‘Well the doctors should check the side-effects shouldn’t they?’ A case of Nitrofurantoin-induced liver injury
Louise Macdougall, Kate Armitage, Richard Thomson and Robert Stirling
Cite this article as: BJMP 2011;4(3):a429
|
|
Abstract This case report discusses an interesting case of drug- induced autoimmune hepatitis following the long term use of nitrofurantoin for recurrent urinary tract infections (UTIs). Recurrent UTIs are common and the evidence for long term antibiotics are discussed along with the difficulty in deciding whether drugs are implicated as the cause of deranged liver function tests. |
An 80-year old lady was referred to a gastroenterology clinic in August 2009 with deranged liver function tests; alkaline phosphatase 180 IU/L (35-120), alanine transferase 147 IU/L (<40), gamma glutamyl transferase 384 IU/L (<45) and globulins 45 g/L (20-35). She had initially presented to her general practitioner with symptoms of lethargy and malaise four months previously. She denied any symptoms of obstructive jaundice and there were no risk factors for hepatitis; she seldom consumed alcohol.
Past medical history included osteoarthritis, migraines and recurrent urinary tract infections; these had been investigated by urology and the patient had undergone cystoscopy and urethral dilatation in September 2003; despite this she continued to experience urinary tract infections and was therefore commenced on prophylactic nitrofurantion by her General Practitioner with approval by the Urologist. This was initially commenced at 50mg at night. This regime was continued for approximately three years however during this time she had a further three treatment courses of nitrofurantoin. In October 2005 her prophylactic dose was therefore increased to 100mg at night. Other medication included lansoprazole 30mg daily, pizotifen 500 micrograms at night, metoprolol 100mg twice daily, simvastatin 10mg at night, senna 15mg at night and furosemide 40mg daily.
On examination there was evidence of palmar erythema and Dupuytren’s contractures but no other stigmata of chronic liver disease. The liver was tender and palpable 4 cm below the costal margin. A liver ultrasound was performed which was normal. Liver screen and autoimmune profile are shown in table 1; notably a positive nuclear antibody was found (1 in 1280 IgG) with Hep 2 cell staining showing a homogenous (ANA) pattern at 1:320 IgG, and a nuclear lamin pattern at 1:1280 IgG;. Due to the positive ANA and raised globulins a suspected diagnosis of nitrofurantoin-induced autoimmune hepatitis was made and a liver biopsy performed.
| Test | Result |
| Hepatitis C antibody | Negative |
| Hepatitis B surface antigen | Negative |
| Ferritin | 85 ug/L (15-300) |
| Caeruloplasmin | 0.33g/L (0.19-0.71) |
| Double-stranded DNA antibody | 4.44 IU/ml (<10) |
| Nuclear antibody | 1 in 1280 IgG |
| Mitochondrial antibody | Negative |
| Smooth muscle antibody | Negative |
| Reticulin antibody | Negative |
| ENA- Ro/La/RNP/Sm/Jo-1/Scl-70 | Negative |
| Liver kidney microsomal antibody | Negative |
| Soluble liver antigen antibody | Borderline |
| Liver cytosol antibody | Negative |
Table 1: liver screen and autoimmune profile
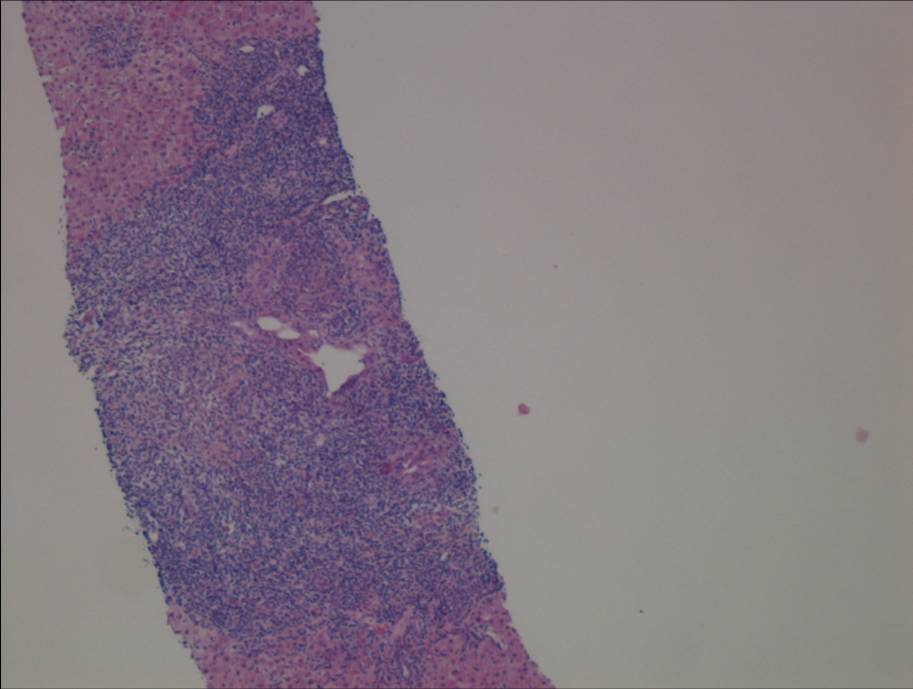
Figure 1a. Liver biopsy showing portal-based interface hepatitis
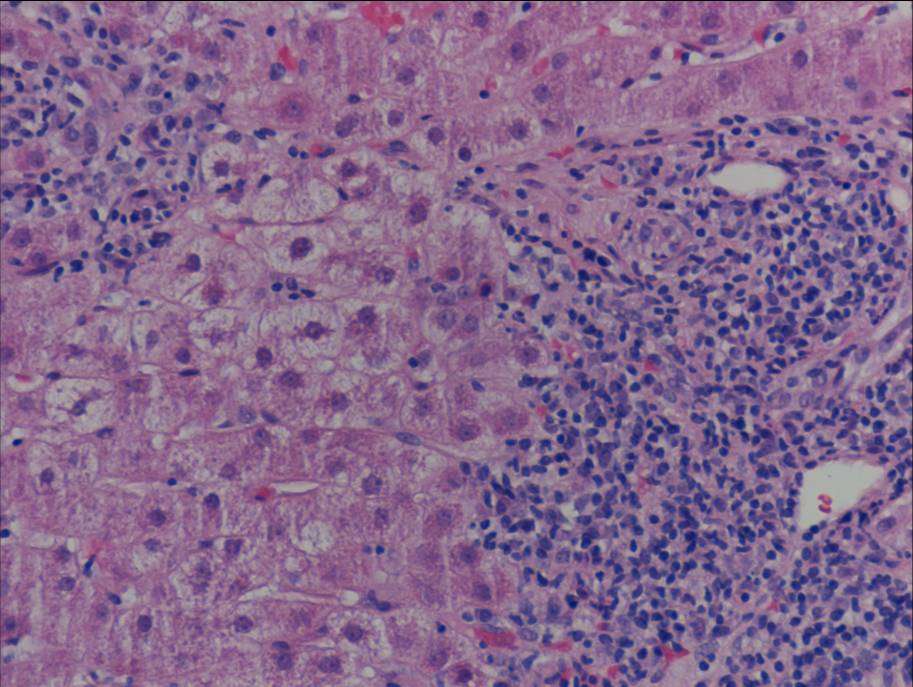
Figure 1b. Liver biopsy showing portal-based interface hepatitis
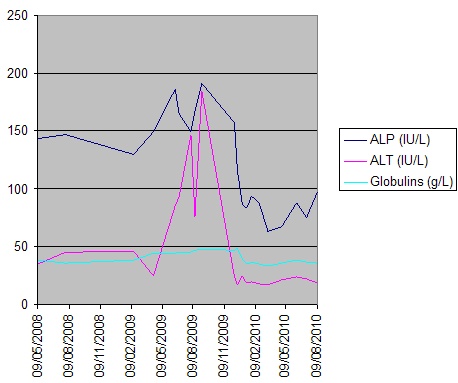
Figure 2. Serial LFTs over time
Liver biopsy (figure 1) indicated a moderate hepatitis which was mainly portal based with multifocal interface hepatitis; these morphological appearances were consistent with those of an autoimmune hepatitis. The patient was advised to immediately stop nitrofurantoin and was commenced on prednisolone 30mg which caused a rapid improvement in LFTs (figure 2). This improvement was maintained following a step-wise reduction in steroid dose and prednisolone was discontinued after eight months of treatment. LFTs are currently normal one month following cessation of steroids
Discussion
This case raises two points of discussion. The first is whether the long term use of nitrofurantoin as prophylaxis for urinary tract infections is appropriate and based on solid evidence. Nitrofurantoin has many side effects and is well documented to cause liver derangement1,2,3. The patient described in this case had been taking nitrofurantoin for seven years and had received a large cumulative dose, on the basis that this was effective prophylaxis. The continuous, long term use of antibiotics as prophylaxis for urinary tract infections is debatable. Madersbacher et al4 recommend the use of prophylactic antibiotics but only after or alongside additional measures including behavioural change, the use of topical oestrogens and the use of alternative therapies; this view is supported by the European Association of Urology5.A Cochrane Review6 in 2004 found that antibiotic use did decrease the number of urinary tract infections compared to placebo but only for the duration of treatment; antibiotics do not alter the natural history of the underlying condition7. There is no clear evidence for duration of treatment and any trials have only been continued for six or twelve months6. It has been noted that all antibiotics had a worse adverse event profile compared to placebo. There was no consensus as to which antibiotic should be used although nitrofurantoin has been associated with a greater withdrawal rate6. One study8 comparing nitrofurantoin and trimethoprim revealed no significant difference in recurrence rates or side effects between the two antibiotics, although this involved a lower dose of nitrofurantoin than was used in this case, and a treatment duration of just 6 months. We would argue that due to the side effect profile of nitrofurantoin and the evidence base available, it is not appropriate to continue it for a duration beyond 6 months.
The second discussion point is whether nitrofurantoin was actually the cause for liver derangement in this case. As documented in a recent review article on the diagnosis of drug-induced liver injury, establishing with any certainty whether liver injury is drug induced can be very difficult3. The key issues are whether there is a temporal relationship between the drug and the onset of liver injury, and whether other causes have been excluded. In this case the patient had negative viral serology and a normal ferritin and caeruloplasmin but her positive autoantibodies raise the possibility of autoimmune hepatitis. Guidelines from the American Association for Liver Diseases9 suggests that the diagnosis of autoimmune hepatitis should be made on the following criteria
- laboratory abnormalities (serum AST or ALT, and increased serum total IgG or gamma-globulins)
- positive serological markers including ANA,SMA, anti-LKM1 or anti- LC1
- histological changes consistent with autoimmune hepatitis i.e. interface hepatitis
This case meets these above criteria for autoimmune hepatitis however the presence of nitrofurantoin does confound the issue. Other case reports10 have reported nitrofurantoin to have caused autoimmune hepatitis based on the relationship between the timing of the drug and the onset of biochemical abnormalities. Bjornsson et al11 performed a comparative study of patients with autoimmune hepatitis and found drugs, particularly nitrofurantoin and minocycline were causally implicated in 9% of cases. When they compared the two groups no significant differences were found in the diagnostic parameters of biochemical, serological and histological abnormalities. In fact the only difference was that no drug-induced cases relapsed on withdrawal of steroids whereas nearly two third of those with non-drug-induced hepatitis relapsed. Bjornsson et al therefore argue in favour of autoimmune immune hepatitis being induced by drugs such as nitrofurantoin; rather than particular drugs simply unmasking sporadic cases based on these management differences.
The patient in this case so far has shown no signs of relapse following steroid withdrawal. We believe that this case does represent one of nitrofurantoin-induced autoimmune hepatitis. In view of the above we would urge readers to consider their use of nitrofurantoin for recurrent urinary-tract infection prophylaxis.
|
Competing Interests None declared Author Details LOUISE MACDOUGALL, Teaching and Research Fellow, North Tyneside General Hospital, UK. KATE ARMITAGE, Teaching and Education Fellow, North Tyneside General Hospital, UK. RICHARD THOMSON, Consultant Gastroenterologist and Clinical Subdean, North Tyneside General Hospital, UK. ROBERT STIRLING, Consultant Histopathologist, North Tyneside General Hospital, UK. CORRESPONDENCE: LOUISE MACDOUGALL, Teaching and Research Fellow, Northumbria Healthcare NHS Trust, North Tyneside General Hospital, Rake Lane, North Shields, Tyne and Wear, NE29 8NH Email: lmacdougall@doctors.org.uk |
References
- Haukekeete ML Hepatoxicity of antibiotics. Act Gastroenterologica Belgica 2003 ; 58 (3): 290-6
- Chalasani N, Fontana R. Causes, clinical features and outcomes from a prospectve study of drug-induced liver injury in the United States 2008;135:1924-34
- Verma S, Kaplowitz Diagnosis, management and prevention of drug-induced liver injury 2009;58: 1555-1564
- Madersbacher S, Thalhammerb F and Marbergera MPathogenesis and management of recurrent urinary tract infection in women Current opinion in Urology 2000; 10:29-33
- Grabe M., Bjerkland-Johansen T.E., Botto H. Guidelines on Urological Infections. European Association of Urology 2010. Available at http://www.uroweb.org/gls/pdf/Urological%20Infections%202010.pdf(Accessed December 2010)
- Albert X, Huertas I, Pereiro II et al. Antibiotics for preventing recurrent urinary tract infection in non-pregnant women. Cochrane Database Syst Rev 2004(3):CD001209
- Madersbacher S, Thalhammerb F and Marbergera MPathogenesis and management of recurrent urinary tract infection in women Current opinion in Urology 2000; 10:29-33
- Vahlensick W Jr, Westenfelder M Nitrofurantoin versus trimethoprim for low dose long-term prophlaxis in patients with recurrent urinary tract infections. A prospective randomized study. International Journal of Urology and Nephrology 1992; 24(1): 3-10
- Manns M.P., Czaja A.J., Gorham J.D. et al Diagnosis and Management of Autoimmune Hepatitis. Hepatology 2010; 51(6): 1-31
- Koulaouzidis A., Bhat S., Moshos J. et al. Nitrofurantoin-induced lung and hepatotoxicity. Annals of Hepatology 2007; 6(2): 119-121
- Bjornsson E., Talwalkar J, Treeprasertsuk S. et al Drug-induced autoimmune hepatitis: clinical characteristics and prognosis. Hepatology 2010; 51 (6): 2040-8

The above article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.




