Tumefactive Multiple Sclerosis Masquerading as Cancer
Kamran Khan, Susan E. Wozniak, JoAnn Coleman
Cite this article as: BJMP 2015;8(4):a832
|
|
Abstract Tumefactive Multiple Sclerosis (MS) is a rare variant of MS that is extremely difficult to diagnose. It can resemble malignancy and perplex the clinician until all diagnostic tests are exhausted. A brain biopsy is not required to treat for the disease, as it is in CNS malignancy. Newer diagnostic tests are available that allow diagnosis to be attained and treated presumptively. Presented is a case of a 48-year-old female that mimicked metastatic malignancy. We were able to use surveillance MRI and CSF analysis to diagnose our patient. Keywords: Tumefactive Multiple Sclerosis, MS, Demyelinating lesionAbbreviations: ECG- Electrocardiogram, CSF- Cerebrospinal Fluid, CN- Cranial Nerve, V/Q- Ventilaion/Perfusion, CA- Cancer Antigen, AFP- Alpha Feto Protein, |
Introduction
Multiple Sclerosis (MS) is an inflammatory, demyelinating disease of the central nervous system (CNS) that affects approximately 400,000 people in the United States and 2.5 million worldwide.1 There are rare variants of this disease that can profoundly delay diagnosis and treatment. Examples of such variants include: Tumefactive MS, Acute Disseminated Encephalomyelitis, Neuromyelitis Optica, Marburg’s MS and Balo Concentric Sclerosis.2 These variants have a uniquely aggressive presentation and do not exhibit classic MS features.2 Classic MS features include relapsing and remitting sensory and motor impairments, optic neuritis and pain. These aggressive variants are more likely to present with symptoms similar to neoplasm such as motor impairments and seizures. When dealing with these aggressive MS variants diagnostic options include Magnetic Resonance Imaging (MRI), Single Photon Emission Computed Tomography (SPECT) scan, MR spectroscopy and Cerebrospinal Fluid (CSF) analysis.3
Invasive tests such as brain biopsy are not warranted unless absolutely necessary. In MS, a biopsy must not be completed in order to confirm a diagnosis. However, to confirm a diagnosis of cancer a biopsy is required.
We present a rare case of Tumefactive MS that exhibited a clinical picture identical to brain metastasis. This was diagnosed with surveillance MRI and CSF analysis in the absence of a brain biopsy.
Case presentation
A 48-year-old African American female was brought in by emergency medical services after falling with a brief loss of consciousness. Associated symptoms included dull chest pain, diaphoresis and shortness of breath. While in the emergency department she also developed nausea, vomiting and dizziness. The patient reported no similar previous episodes and denied precipitating events. There was nothing else to note on review of systems. The past medical history included hypertension with no previous surgeries and family history included breast cancer of the mother diagnosed at age 47. The patient denied tobacco, alcohol and intravenous drug use. She noted an allergy to iodine.
On physical examination the patient was afebrile, normotensive and tachycardic with an oxygen saturation of 89% on room air. She was alert and oriented but pale and diaphoretic with mild left sided chest pain. Cardiac examination revealed a normal rhythm tachycardia and no murmurs were heard. Her neurological examination showed a normal mental status, normal cognition/comprehension and that Cranial Nerves II-XII were intact.
Laboratory findings included haemoglobin of 9.8 g/dL, 30.8% haematocrit and potassium of 3.3 mmol/L. Electrolytes were otherwise normal. Cardiac workup showed a normal ECG and slightly elevated cardiac enzymes of 0.319 ng/mL.
Given the patients tachycardia and desaturation, a stat Ventilation-perfusion (V/Q) scan was completed (patient had an iodine allergy). The V/Q scan revealed a perfusion defect suggesting pulmonary embolism (PE) as the cause of symptoms. Subsequently the patient was placed on appropriate anticoagulation.
Head CT (computed tomography) showed a left centrum semiovale round hypodense lesion measuring 1.4 cm, a left basal ganglia round hypodense lesion measuring 1.0 cm and a left occipital lobe round hypodense lesion measuring approximately 1.0 cm (Figure 1). No midline shift was seen. MRI showed multiple hypointense T1/hyperintense T2 nonenhancing lesions, mainly within the left cerebrum (Figure 2 A-F). The three largest lesions within the left posterior centrum semiovale (2A), left globus pallidus (2B) and left posterior corona radiata adjacent to the occipital horn (2C) measured 1.5 cm, 1.0 cm and 1.0 cm respectively. Perilesional vasogenic oedema was seen in all except the basal ganglia lesion. There were bilateral cerebral scattered foci of hyperintense FLAIR/T2 signals (D-F). The imaging suggested a differential diagnosis which included metastasis, infection or primary CNS malignancy.
Further work up in search for possible malignancy was completed. Skin map revealed no concerning nevi. Mammogram showed no tumor. CT of the abdomen and pelvis revealed a 2.6 cm indeterminate hypodense lesion in the left lobe of the liver (Figure 3A) along with an enlarged fibroid uterus (17x 7 x 14 cm). Liver biopsy was considered but a repeat MRI and ultrasound showed the lesion to be cystic, so this was deferred following surgical oncology recommendations (Figure 3B). For the hypertrophic uterus found on imaging, gynecology felt no further workup was necessary as they attributed the findings to a fibroid uterus.
Tumor markers CA 27-29, CA 19-9, CA 125 and AFP were all sent and came back negative. Initial lumbar puncture with CSF analysis was not completed secondary to possible complications that could be incurred while on necessary PE anticoagulation.
Due to a non-focal neurological examination, she was discharged on Levetiracetam 500 mg for seizure prophylaxis and Dexamethasone 4 mg for perilesional oedema. Over subsequent months the patient did well without headaches, vision changes or seizure like activity. On subsequent visits to the clinic, she had no evidence of focal neurological deficits except for mild bilateral symmetric hyperreflexia. Given that the metastatic work up remained negative, we considered obtaining a baseline Positron emission tomography (PET) scan to ensure we were not missing any possible metastasis.
She subsequently went back to work full-time and reported no symptoms. Repeat MRI of the head (Figure 4 A-C) showed predominantly T1 hypointense and T2 hyperintense (A-B) lesions with significant decrease in size from MRI done three months ago. These lesions demonstrated no enhancement to incomplete ring enhancement, with diminished vasogenic oedema (A). These findings suggested an inflammatory demyelinating process so a lumbar puncture was obtained after anticoagulation was held. CSF analysis was done using Isoelectric Focusing (IEF) and immunoblotting methodology. This revealed a normal myelin basic protein but with eight oligoclonal bands restricted to the CSF. These findings solidified the suspicion of Tumefactive MS.
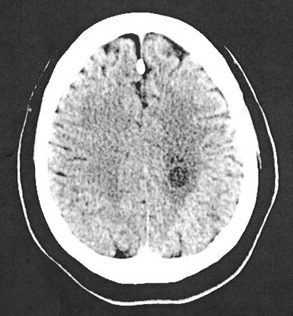
Figure 1. Head CT without contrast: left centrum semiovale round hypodense lesions measures 1.4 cm with perilesional vasogenic edema
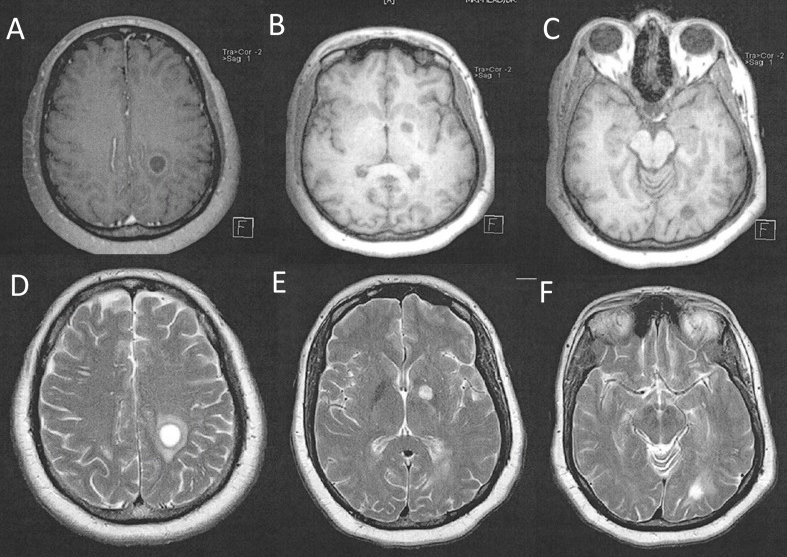
Figure 2. MRI of brain showing axial T1-weighted (A-C) hypodense lesions of the left centrum semiovale(A), left basal ganglia(B) and left occipital lobe(C). Axial T2-weighted (D-F) views show multiple hyperdense lesions corresponding to the same locations. Perilesional vasogenic edema is seen.
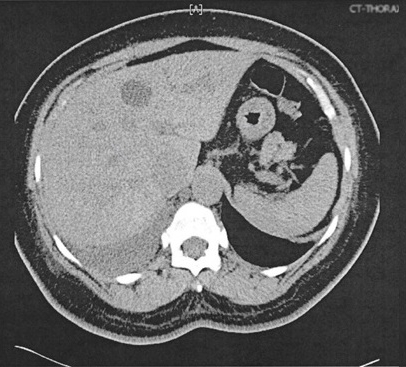
Figure 3A. Thorax CT without contrast. 2.6 cm left lobe liver lesion.
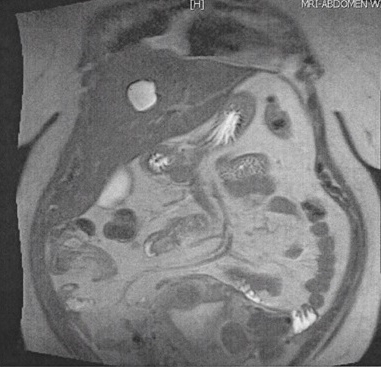
Figure 3B. MRI of abdomen showing coronal T2-weighted half-Fourier acquisition single-shot turbo spin-echo (HASTE) hyperdense lesion. A mildly enlarged liver measuring 18.7 cm in craniocaudal span. Simple 2.8 x2.4 cm cyct in the medial segment of left lobe.
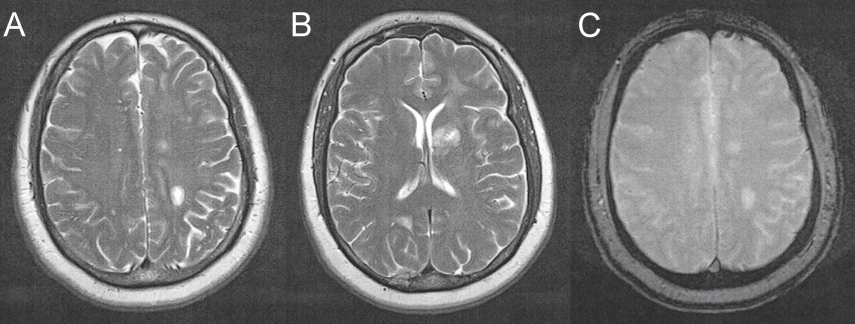
Figure 4. MRI of brain (3 month after initial scans) showing axial T-2 weighted (A-B) hyperdense lesions of the left centrum semiovale(A) and left basal ganglia(B). There is irregular peripheral enhancement. Considerable decrease in size is seen from previous MRI (Figure 2). Left posterior centrum semiovale, left globus pallidus and left occipital lobe lesion measure 1.3 cm, <1 cm and <1 cm respectively. Vasogenic edema is diminished in comparison to previous study.
Discussion
Tumefactive MS lesions are defined as solitary demyelinating plaques greater than 2 cm.5 Lesions are difficult to distinguish between primary or metastatic given similarity of imaging features.5 Imaging features suggestive of Tumefactive MS include incomplete ring enhancement, absence of mass effect and absence of cortical involvement.6 7 Kim describes that CT hypoattenuation of magnetic resonance enhancing lesions was found to be highly specific for distinguishing Tumefactive MS lesions from CNS cancer pathology.6 It has been shown that SPECT using I-IMP is useful for diagnosing CNS malignancy.3 This is because there would be increased uptake in comparison to the MS lesions - implying increased metabolic activity.3 However this study has its limitations in diagnosis. In a few isolated cases I-IMP was found in greater quantities in MS tumor-like lesions.3
The imaging studies for this patient established a concern for metastasis, infection or primary malignancy. Extensive cancer workup was completed as previously discussed. Since all tumor markers were negative a baseline PET scan was considered however, was not done secondary to insurance denial. Due to the asymptomatic presentation of her disease, a primary differential diagnosis of brain metastasis and anticoagulation therapy for PE, a CSF analysis was not considered until much later. We were able to use surveillance MRI and CSF analysis to see some resolution of these lesions and confirm the diagnosis. Brain biopsy was never warranted but in unique symptomatic cases it may have been.6
The cornerstone of diagnosing MS is the demonstration of lesions in both time and space - termed the McDonald Criteria.8 The revised criteria allow a diagnosis of MS, “possible MS” or “not MS”.8 This is what made the diagnosis of our patient difficult, as no clinical symptoms or attacks were evident. It was demonstrated that over the course of three months the lesions seen on MRI evolved. From the size of 1.5 cm, 1.0 cm and 1.0 cm they became 1.3 cm, <1.0 cm and <1.0 cm respectively (Figure 2, Figure 4). This was likely the effects of steroids that the patient was on due to her vasogenic oedema. Here an evolution in time and space is demonstrated which excluded brain metastasis and infection. This brings into discussion the diagnostic value of surveillance MRI, which in our case was helpful and appropriate as the patient did not have clinical symptoms.
Conclusion
The diagnosis of Tumefactive MS can be extremely difficult and time consuming. As seen in our case, it can mimic other conditions. Our patient was able to be diagnosed with MRI surveillance and CSF analysis. The definitive diagnostic test for MS is a brain biopsy but this is not preferred due to the invasiveness of the procedure. With the advent of newer diagnostic tests such as SPECT, MR Spectroscopy, surveillance MRI and CSF analysis, diagnosis can be attained and treated presumptively.
|
Acknowledgements We would like to acknowledge Dashartha Harsewak MD for interpreting radiological scans, Musarat Shareeff MD for valuable guidance and Anna Lucia Giannone for input on figure design. Competing Interests None declared Author Details KAMRAN KHAN, Medical Student, Sinai Hospital, Baltimore, MD, USA. SUSAN E. WOZNIAK, MD, MBA, General Surgery Resident, Sinai Hospital, Baltimore, MD, USA. JOANN COLEMAN DNP, ANP, ACNP, AOCN, Acute Care Nurse Practitioner & Clinical Program Coordinator, Sinai Center for Geriatric Surgery, Baltimore, MD, USA. CORRESPONDENCE: KAMRAN KHAN, Sinai Hospital, Baltimore, MD, USA. Email: kamkmd92@gmail.com |
References
- Hersh CM, Fox RJ. Multiple Sclerosis [Internet]. Clevelandclinicmeded.com. 2014 [cited June 2015] Available from: http://www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/neurology/multiple_sclerosis/
- Hamed SA. Variant of multiple sclerosis with dementia and tumefactive demyelinating brain lesions. World J clin cases. 2015 June 16; 3(6): 525-532
- Sagiuchi T, Oka H, Utsuki S. Increased accumulations of N-isopropyl-p-[123I]- iodoamphetamine related to tumefactive multiple sclerosis. Annals of Nuclear Medicine Vol. 2005;19,No. 7;603-606,
- Yamada S, Yamada MS, Nakaguchi H. Tumefactive multiple sclerosis requiring emergent biopsy and histological investigation to confirm the diagnosis: a case report. Journal of Medical Case Reports. 2012;6:104
- Fallah A, Banglawala S. Ebrahim S. Tumefactive demyelinating lesions: a diagnostic challenge. Can J Surg. 2010;53, No. 1
- Jitawatanarat P, Tingpej B, Deringer P. Tumefactive Multiple sclerosis. BJMP. 2011;4(2):a419
- Kim DS, Na DG, Kim KH. Distinguishing tumefactive demyelinating lesions from glioma or central nervous system lymphoma: added value of unenhanced CT compared with conventional contrast-enhanced MR imaging. Radiology. 2009 May;251(2):467-75.
- McDonald WI, Compston A, Edan G. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001 Jul;50(1):121-7.

The above article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.




