Trend of developing resistance among isolates of Acinetobacter spp.; Threat of hospital acquired infection
Sadia Zafar, Syed Baqir Shyum Naqvi, Tanveer Abbas, Faaiza Qazi and Rabia Sheikh
Cite this article as: BJMP 2015;8(1):a802
|
|
Abstract Aim: Acinetobacter sp. is a Gm-ve bacteria which is a major cause of serious infections. Today it has emerged as multidrug resistant organism. The aim of current study was to evaluate the trend of sensitivity/resistance pattern of Acinetobacter spp. against broad spectrum antibiotics. Abbreviations: CLSI :Clinical and Laboratory Standards Institute |
Introduction
For decades the genus Acinetobacter has undergone several taxonomical modifications. Large number of non-fastidious, aerobic, Gram-negative bacteria (GNB) are included in this genus. In the last few years these organisms are genetically modifying into highly resistant forms resulting in untreatable nosocomial infections1 and health care associated infections.2 Acinetobacter is also a major cause of invasive type infections in children resulting in untreatable urinary tract infections (UTIs), skin infections and septicemia.3 One identified cause of the resistance mechanism in carbapenem resistant Acinetobacter spp. is the production of the MBL enzyme.4 It has been revealed through various published studies that Acinetobacter displays a specific type of mechanism of resistance against different antimicrobials. Some of them, for example β-lactam, are inhibited by enzymatic degradation, while quinolones are rendered ineffective due to a genetic mutation preventing the binding of an antibiotic to a distinct binding site. The same is true with aminoglycosides in which the resistant strains are noticed to acquire a gene involved in enzymatic modification.1
Although polymixin resistance in Acinetobacter spp. was reported the specific cause of resistance was unknown until 2008. In 2013, one study detected the presence of hetero-and adaptive resistance due to mutation in specific gene for the first time.1,21 Hence the aim of this current study was to evaluate the trend of sensitivity/resistance pattern of Acinetobacter spp. against broad spectrum antibiotics.
Method and Materials
The objective of the study was to evaluate the sensitivity of Acinetobacter spp. to 08 broad spectrum antibiotics. The Kirby Bauer Disc Diffusion method was used following the standard procedures as laid down by CLSI 2013.6 A total of 52 isolates were collected from Feb 2014-March 2014 from patients admitted to tertiary care hospitals in Karachi. The isolates were identified by routine lab procedures.
Antimicrobial agents and medium: Standard (Oxoid) discs of Amikacin (30 µg), Cefoperazone (75 µg), Ceftriaxone (30 µg), Ciprofloxacin (5 µg), Colistin (10µg), Fosfomycin (50 µg), imipenem (10 µg), Polymixin B (300units), Mueller Hinton Agar (Oxoid UK) and Mueller Hinton broth (Oxoid UK) were used.
0.5 McFarlan Standard: The inoculum was grown at 370C for 2-6 hrs. Turbidity Standard of 0.5 McFarland was achieved by incubating broth culture.
Inoculation of test plates:The plates were inoculated with the culture of Acinetobacter spp. by the help of sterile cotton swabs. The excess fluid was removed after the cotton swab was dipped into inoculum suspension. When the inoculum were dried the antibiotic discs were placed with sterile forceps onto the agar surface.15
Incubation of test plates: The isolates after application of antibiotic discs plates were incubated for 24 hours and results were interpreted according to CLSI standards 5,6. Interpretative standards for used antibiotics and Zone diameter of inhibition are shown in Table 25.
Control strain: Escherichia coli ATCC 25922was used as a control strain to maintain accuracy and precision of procedures.
Results
It is reported that out of all the samples 61.5% were obtained from male patients. Infections caused by Acinetobacter spp. had a high prevalence among both the genders among the age group 51-75 yrs. The most frequent site of isolate collection was tracheal aspirate (55.76%) among both genders and the second highest percentage of isolate was obtained from sputum (19.23%) as shown in Table 1. The Colistin and Polymixin B were found equally effective against Acinetobacter spp. by inhibiting 98% of isolates each and 19.23% isolates showed sensitivity against Amikacin. The isolate showed the highest degree of resistance against Imipenem (98%), followed by Cefoperazone (94.23%) and Ceftrioxone (92.3). Surprisingly 32.69% of isolates exhibited intermediate sensitivity (IS) against Fosfomycin as indicated in Table 3 and Figure 1.
Table 1: Age and gender specific distribution of Acinetobacter spp. among patients
| Age | Male n=32(61.5%) | Female n=20(38.46%) |
| 00-25 | 10 | 06 |
| 26-50 | 05 | 02 |
| 51-75 | 12 | 11 |
| 76-100 | 05 | 01 |
Table 2: Zone diameter interpretive standards for Acinetobacter spp. CLSI standards table of antibiotics for Acinetobacter spp.
| Antibiotic | Disc Content | Zone of Inhibition (mm) | ||
| Resistance | Intermediate | Sensitive | ||
| Amikacin | 30µg | ≤14 | 15-16 | ≥17 |
| Cefoperazone | 75 µg | ≤15 | 16-20 | ≥21 |
| Ceftriaxone | 30 µg | ≤13 | 14-20 | ≥21 |
| Ciprofloxacin | 5 µg | ≤15 | 16-20 | ≥21 |
| Colistin٭ | 10µg | ≤11 | ≥17 | |
| Fosfomycin٭ | 50 µg | ≤12 | 13-15 | ≥16 |
| Imipenem | 10 µg | ≤13 | 14-15 | ≥16 |
| Polymixin B٭ | 300units | ≤13 | ≥19 | |
*Since the interpretive standards for Colistin, Fosfomycin and Polymixin B against Acinetobacter spp. is not established in CLSI 2013 mannual zone diameter interpretative standards for Enterobacter spp. and E. coli were used.20
Figure 1: Susceptibility pattern of Acinetobacter spp. against broad spectrum antibiotics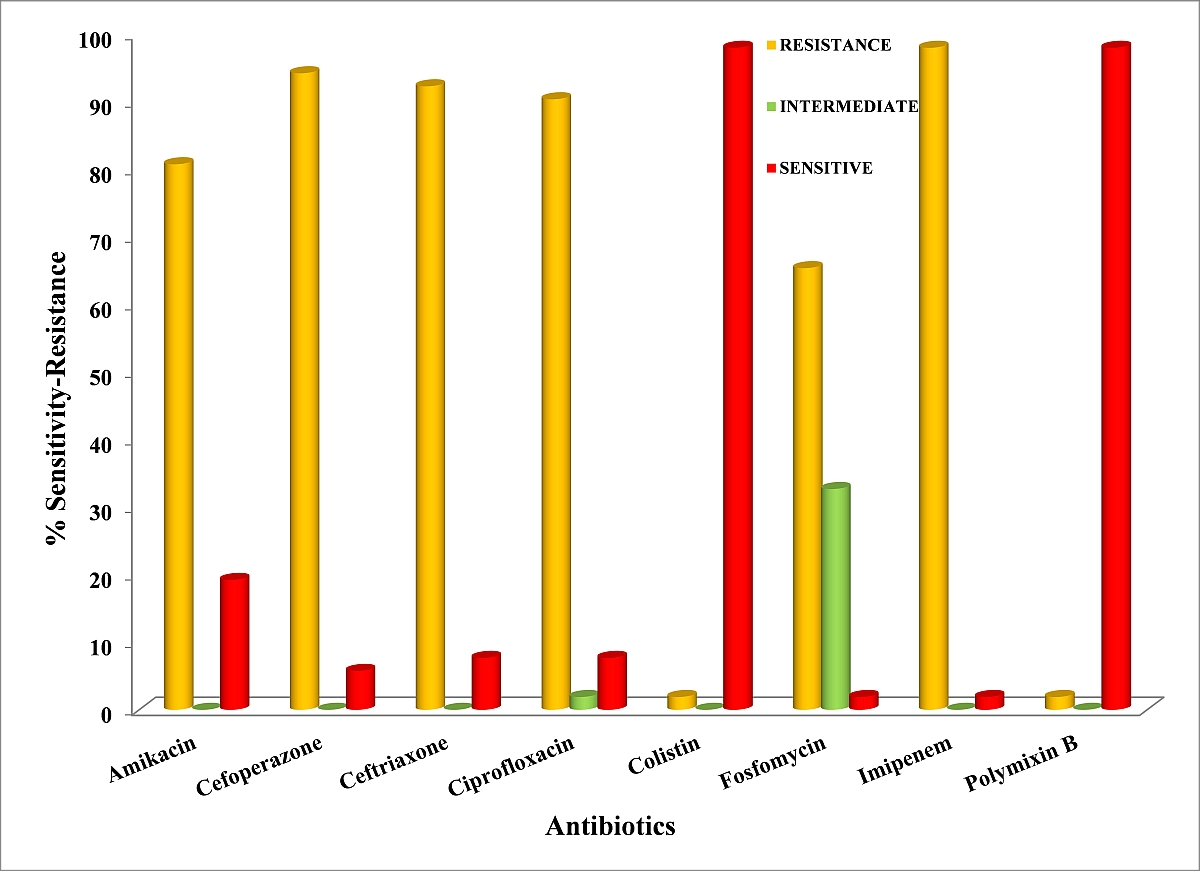
Table 3: Total % efficacy of different antibiotics among Acinetobacter spp. isolated (N= 52)
| S.No. | Antibiotics | Disc Code | Resistance (%) | Intermediate (%) | Sensitivity (%) |
| 1. | Amikacin | 30µg | 42(80.76) | 00 | 10(19.23) |
| 2. | Cefoperazone | 75µg | 49(94.23) | 00 | 03(5.76) |
| 3. | Ceftriaxone | 30µg | 48(92.3) | 00 | 04(7.69) |
| 4. | Ciprofloxacin | 05µg | 47(90.38) | 01(1.9) | 04(7.69) |
| 5. | Colistin | 10µg | 01(1.9) | 00 | 51(98) |
| 6. | Fosfomycin | 50µg | 34(65.38) | 17(32.69) | 01(1.9) |
| 7. | Imipenem | 10µg | 51(98) | 00 | 01(1.9) |
| 8. | Polymixin B | 300 units | 01(1.9) | 00 | 51(98) |
Discussion
Our present study shows that the Acinetobacter spp. were highly resistant to Cefoperazone (94.23%). This finding is further substantiated by research that observed Cefoperazone to be only effective when used in combination.7,8
We also observed that only 19% isolates were sensitive to Amikacin, which contradicts the findings of Liu et al 2013 3 who observed 100% efficacy. However, they also discovered that 82% were inhibited by Imipenem while Fluoroquinolone was also found to be effective against 70% of all isolated organisms and Cefoperazone as least effective.
Organisms isolated from sputum showed a high degree of resistance to most of antibiotics, Zheng W and Yuan S also observed such results9.Nwadike et al 201410 found a high prevalence of resistant Acinetobacter spp. isolates against Ciprofloxacin (100%) and Amikacin (50%).10
Polymixin inhibited 98% of isolates, which is similar to figures found by Haeili et al 20131 who observed 95.5% susceptibility to Polymixin B. The second most effective antibiotic was Colistin - Trottier et al 200712 also observed 100% susceptibility of A. baumanni to Colistin. Similarly, Vakilietal 201413 found a low rate (i.e, 11.6%) of Colistin resistance.
Colistin has emerged as a viable choice for treatment of multidrug resistant Acinetobacter strains. In several studies,13,14 where 98% of isolates were resistant to Imipenem these results support the work of Khajuria et al 201416 who also reported reduced efficacy. Our findings are in contradiction to the study by of Tripathi et al 201417 who reported that Imipenem was a highly effective drug in comparison to other broad spectrum antibiotics.Fosfomycin surprisingly exhibited unusual results in our study; 32% of Acinetobacter spp. were IS while 65% were resistant. However, previous studies showed that Fosfomycin were proved to be good option to treat infections caused by Acinetobacter spp.18 Zhang et al 201319 reported that Fosfomycin used alone was highly ineffective in treatment of Penicillin Drug Resistant-Acinetobacter baumannii (PDR-Ab).Another study revealed that Acinetobacter spp. has developed adaptive resistance against Polymixin.21
Acinetobacter spp. are emerging as a resistant bacteria and a common cause of nosocomial and hospital acquired infections. There is a serious need to take necessary measures by hospital administration in maintaining environmental and personnel cleanliness according to current Good Manufacturing Practices. Pharmacists should educate patients about the drawbacks of self-medication and not completing medication courses, which is resulting in development of resistant bacterial pathogens.
|
Competing Interests None declared Author Details SADIA ZAFAR , M.PHIL, Faculty of Pharmacy, Jinnah University for Women, Karachi, 5C, Nazimabad, Karachi , PAKISTAN. SYED BAQIR SHYUM NAQVI, PHD, Department of Pharmaceutics, Faculty of Pharmacy, University of Karachi,Main university road, PAKISTAN. TANVEER ABBAS, PHD, Department of Microbiology, University of Karachi,Main university road, PAKISTAN. FAAIZA QAZI, M.PHIL, Faculty of Pharmacy, Jinnah University for Women, Karachi, 5C, Nazimabad, Karachi , PAKISTAN. RABIA SHEIKH, B.PHARM, Nigehban Compounding Pharmacy, Karachi,Bangalow#46 C.P.Brar society, PAKISTAN. CORRESPONDENCE: SADIA ZAFAR,MUHAMMAD ZAFAR IQBAL, FACULTY OF PHARMACY, JINNAH UNIVERSITY FOR WOMEN, KARACHI, P.O.Box 74600, PAKISTAN. Email: sadiazafarnew@yahoo.com |
References
- Peleg AY, Seifert H and Paterson DL. Acinetobacter baumannii: Emergence of a Successful Pathogen Clin. Microbiol. Rev.2008, 21: 3 538-582
- Tekin R, Dal T, Pirinccioglu H, et al A 4-year surveillance of device-associated nosocomial infections in a neonatal intensive care unit.PediatrNeonatol. 2013,54:303-8.
- Liu L, Dong L, Xu YB, Chen ZX, et al Clinical characteristics and antibiotic resistance in children with invasive Acinetobacter baumannii infection.Zhongguo Dang Dai ErKeZaZhi. 2013,:15:379-82.
- Zhao C, Xie W, Zhang W, Ye Z, et alMechanism of drug resistance of carbapenems-resistant Acinetobacter baumannii and the application of a combination of drugs in vitro.Zhonghua Shao Shang ZaZhi. 2014, 30:166-70
- Lorian V. (1991). Antibiotics in Laboratory Medicines, 3rd edition.Williams & Wilkins Publ. pp.18, 17, 906.
- Bauer A.W, Kirby W.M.M., Sherns J.C. and Turck M. Antibiotic suscepti- bility testing by a standardized single disk method.Am. J. Clin. Pathol. 1966, 45: 493.
- Ning F, Shen Y, Chen X, et al A combination regimen of meropenem, cefoperazone-sulbactam and minocycline for extensive burns with pan-drug resistant Acinetobacter baumannii infection.Chin Med J (Engl). 2014,127:1177-9.
- Poudyal N, Gyawali N, Gurung R, et al In vitro activity of cefoperazone-sulbactam combination against gram negative bacilli.Nepal Med Coll J. 2012,14:5-8.
- Zheng W, Yuan S, Li L.Analysis of hospital departmental distribution and antibiotic susceptibility of Acinetobacter isolated from sputum samples.Am J Infect Control. 2013,41:e73-6.
- Nwadike VU, Ojide CK, Kalu EI. Multidrug resistant Acinetobacter infection and their antimicrobial susceptibility pattern in a nigerian tertiary hospital ICU.Afr J Infect Dis. 2014,8:14-8
- Haeili M, Ghodousi A, Nomanpour B, et al Drug resistance patterns of bacteria isolated from patients with nosocomial pneumonia at Tehran hospitals during 2009-2011.J Infect DevCtries. 201,7:312-7.
- Trottier V, Segura PG, Namias N,et al Outcomes of Acinetobacter baumannii infection in critically ill burned patients.J Burn Care Res. 2007,28:248-54
- Vakili B, Fazeli H, Shoaei P, et al . Detection of colistin sensitivity in clinical isolates of Acinetobacter baumannii in Iran. J Res Med Sci. 2014,19(Suppl 1):S67-70
- Ece G, Samlioglu P, Atalay S, et al Evaluation of the in vitro colistin susceptibility of Pseudomonas aeruginosa and Acinetobacte rbaumannii strains at a tertiary care centre in Western Turkey.Infez Med. 2014,22:36-40.
- National Commitce for Clinical Laboratory Standards (1990). Tentative Standard M7-A2.Methods for dilution anti- microbial susceptibility tests for bacteria that grow aerobically. National Committee for Clinical Laboratory Standards.,Villinova, Pa
- Khajuria A, Praharaj AK, Kumar M,et al Molecular Characterization of Carbapenem Resistant Isolates of Acinetobacterbaumannii in An Intensive Care Unit of A Tertiary Care Centre at Central IndiaJ ClinDiagn Res. 2014,8:DC38-40.
- Tripathi PC, Gajbhiye SR, Agrawal GN. Clinical and antimicrobial profile of Acinetobacter spp.: An emerging nosocomial superbug. Adv Biomed Res. 2014,3:13.
- 18.Perdigão-Neto LV, Oliveira MS, Rizek CF, et al Susceptibility of multiresistant gram-negative bacteria to fosfomycin and performance of different susceptibility testing methods. Antimicrob Agents Chemother. 2014,58:1763-7.
- Zhang Y, Chen F, Sun E, et al In vitro antibacterial activity of combinations of fosfomycin, minocycline and polymyxin B on pan-drug-resistant Acinetobacter baumannii.ExpTher Med.2013 ;5(6):1737-1739
- GülÖzseven A, Çetin ES, Arıdoğan BC and Özseven L In vitro synergistic activity of carbapenems in combination with other antimicrobial agents against multidrug-resistant Acinetobacter baumannii African Journal of Microbiology Research 2012; 6(12): 2985-2992
- Barin J, Martins AF, Heineck BL,et al Hetero- and Adaptive Resistance to Polymyxin B in OXA-23-Producing Carbapenem-resistant Acinetobacter baumannii Isolates. Ann ClinMicrobiolAntimicrob. 2013;12(15)

The above article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.




