A Registry Comparison of ESC and NICE guidelines 95 in the assessment of stable angina in a UK district hospital
Jessica Ball, Andrew Cai, A Pineau-Mitchell, Katie Brown, Benjamin Coope and Kuno Budack
Cite this article as: BJMP 2016;9(3):a925
|
|
Abstract Background: National Institute for Clinical Excellence (NICE) and European Society of Cardiology (ESC) have developed guidance and risk-stratification tables to assist physicians in assessing the pre-test probability of coronary artery disease (CAD) in patients with stable chest pain. We hypothesised that NICE clinical guideline 95 overestimates prevalence of CAD and that using ESC guidelines instead may enable more targeted, cost-effective use of investigations. Methods and results: Clinic records of 1968 patients who attended Tunbridge Wells Hospital’s Rapid Access Chest Pain Clinic between July 2005 and December 2012 were reviewed. A comparison was made between the pre-test probability of CAD in these patients and the actual incidence of CAD. In patient groups where NICE guidelines’ pre-test probability of CAD was 61–90%, 31-60%, 10-29% and <10%, actual incidence of CAD was 31% (95% CI 27.6 – 34.5), 4.4% (3.0–6.5), 2.5% (1.2-5.0) and 0.28% (0.1–1.6) respectively. Where ESC guidelines pre-test probability of CAD was >85%, 66-85%, 15-65% and <15%, actual incidence of CAD was 73.4% (63.7–82.7), 58.5% (51.1–65.5), 6.4% (5.3–7.8) and 0.76% (0.2–2.7) respectively. Conclusion: Strict adherence to NICE guidelines overestimates the pre-test probability of CAD in our cohort. ESC guidelines offer a more conservative estimate and their use may reduce the number of coronary angiograms performed, resulting in more cost-effective practice. £322,545.88 was spent on investigations when hypothetically applying ESC guidelines to our cohort, compared with £943,865.22 spent when applying NICE guidelines. However, strict use of ESC guidelines may risk missing other diagnoses of chest pain. Keywords: angina pectoris, coronary artery disease, chest pain, risk, pre-test probabilityAbbreviations: NICE - national institute for clinical excellence, ESC - European Society of Cardiology, CAD - coronary artery disease, RACPC - rapid access chest pain clinic |
Introduction
Chest pain accounts for 1% of all GP consultations, but in only 8%-18% of cases is it an indicator of underlying ischemic heart disease.1 Given the potential diagnostic uncertainty associated with chest pain at initial presentation, specialist evaluation of patients in a Rapid Access Chest Pain Clinic (RACPC) is of value and represents an important process in the evaluation of symptoms. These clinics were established with the aim of providing rapid outpatient assessment of patients with suspected cardiac disease in order to permit earlier provision of appropriate treatment and investigations where required.
Stable chest pain typically presents as angina, a triad of dull central chest pain, brought on with exertion and relieved by rest or GTN spray. The aetiology is usually stable atherosclerotic plaque disease which is associated with low mortality and can be treated with oral anti-anginals, as demonstrated by meta-analyses and the landmark COURAGE study. 2, 3
NICE Clinical Guideline 95 (NICE CG95) suggests that choice of initial investigation for stable chest pain should be guided by a patient’s pre-test probability of having CAD. Calculations of the pre-test probability take into consideration a patient’s age, gender, cardiac risk factors and symptoms. Patients are defined as high risk of cardiac disease if they have diabetes, smoke or have hyperlipidaemia (total cholesterol >6.47mmol/litre). Patients with none of the above are considered low risk. Symptoms are defined as “typical angina” if the pain is: 1) constricting discomfort in the front of the chest or in the neck, shoulders, jaw or arms; 2) is precipitated by physical exertion and 3) is relieved by rest or GTN spray within approximately five minutes. Pain is defined as “atypical angina” if only two of the above criteria are met and defined as “non-anginal” if one or none of the above criteria are met.
NICE pre-test probabilities of CAD (Table 1), are based on a version of Diamond and Forrester’s pre-test probabilities published in 1979, modified using data from Duke’s cohort study, published in 1993.4, 5, 6 Recent studies suggest that these NICE pre-test probabilities may overestimate the prevalence of CAD in a primary care population and may risk over investigating patients.7, 8 In addition to having financial implications, this may cause patients undue anxiety and unnecessarily put them at risk of complications.
Table 1: NICE Clinical Guideline 95 pre-test probabilities table.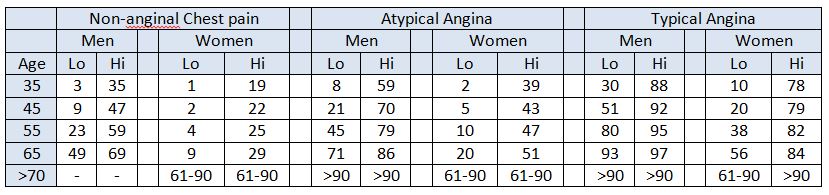
Each cell represents the percentage risk of each group of patients having CAD, based on their typicality of symptoms, gender, age and cardiac risk factors (lo, low and hi, high)4
ESC guidelines utilise an updated, validated model of the Diamond-Forrester model by Genders et al. to create pre test probabilities of CAD (Table 2), based on patient’s age, gender and typicality of symptoms. 9, 10
Table 2: ESC guidelines clinical pre-test probabilities in patients with stable chest pain symptoms 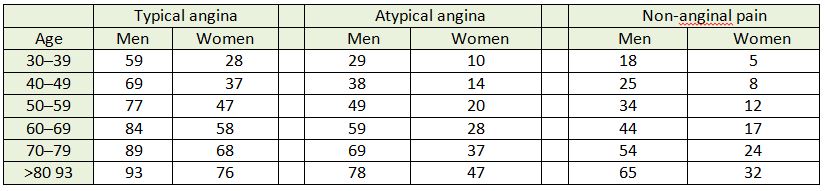
Each cell represents likelihood of each group of patients having CAD, based on typicality of symptoms, age and gender.9
We hypothesised that strict adherence to NICE guidelines results in over-estimation of the pre-test probability of CAD and therefore over-investigation of patients presenting with stable chest pain. ESC guidelines may offer more accurate pre-test probabilities of CAD and allow a more targeted and cost-effective use of investigations.
Methodology
Clinic records of all patients who attended the RACPC at Tunbridge Wells Hospital between July 2005 and December 2012 were reviewed. This service is run by a cardiology specialist. Patient demographics, cardiac risk factors and information regarding the nature of patient symptoms were collected prospectively and completed at the time of the patient’s RACPC appointment. Results of cardiac investigations were collected from paper and computerised records, and included diagnoses of significant CAD made following invasive coronary angiogram. These results were compared with patients’ pre-test probabilities of CAD calculated using both NICE and the ESC’s calculation methods. Outcome and readmissions were obtained from electronic records from the Maidstone and Tunbridge Wells NHS Trust computer records retrospectively.
Results
Study population
A total of 1968 records were reviewed. 59% (n = 1162) of patients were male and 41% (n = 806) were female. Their mean age was 60 years. At initial assessment, 69.8% patients (n=1373) had non-anginal chest pain, 19.5% (n=383) had atypical angina and 10.8% (n=212) had typical angina, based on the NICE guideline definitions of chest pain.
97.2% (n= 1912) patients underwent further investigation; 15% (n=256) of these were subsequently diagnosed as having significant CAD, accounting for their symptoms. The 2.8% (n=56) of patients who did not undergo investigation either chose not to, were unable to, were lost to follow up, or were diagnosed as having a non-cardiac cause of their symptoms at the initial RACPC appointment.
NICE CG95 pre test probabilities compared against cohort data
Table 3: NICE guidelines 95 pre test probabilities compared against cohort data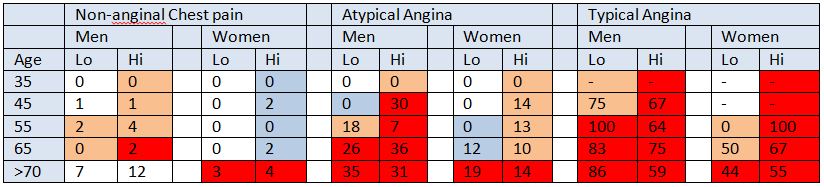
Each cell represents the proportion (%) of cohort patients from each group who were diagnosed with CAD. We have colour-coded cells to represent the NICE estimated pre-test probability of CAD in each group. Red cells represent 61-90+% probability, pink cells represents 30-60% probability, blue cells represent 10-29% probability and white cells represents <10% probability of CAD according to NICE Guidelines. “ – “ marks a cell where pre-test probabilities of CAD could not be calculated for cohort patients.
Table 4: A comparison of NICE pre-test probabilities and cohort patient data.
The risk of CAD as predicted by NICE guidelines 95 on the left compared with the actual number of cohort patients in each category and the proportion of those patients diagnosed with significant CAD.
The average discrepancy between the pre-test probability and actual incidence of CAD in cohort patients was 28% (range 20% - 88%). In 48% of cells in the NICE CG95 pre-test probability table (Table 1) the pre-test probability of CAD was overestimated by 30% or more (Table 3). A marked discrepancy between pre-test probability and actual incidence of CAD was found between “high risk” and “very low risk” patients. On average, high risk patients had an overestimated pre-test probability of 34.3 – 40.9% per cell compared with low risk patients whose pre-test probability was only overestimated by 6.5% (Table 3).
The cells highlighted in dark red in table 3 represent high risk patients whose pre-test probability was of 61-90+%, according to NICE CG95. In our cohort, only 31.2% (n=214, 95% CI 27.6-34.5) of high risk patients in this category were diagnosed with CAD. On average, actual incidence of CAD compared with pre-test probability was overestimated by 34.4% – 40.9% in each cell.
The pink cells in table 3 represent medium risk patients with a pre-test probability of CAD of 30-60%, according to NICE CG95. In our cohort, only 4.4% (n=24, 95% CI 3.0 – 6.5) of medium risk patients had a positive angiogram (Table 4). The average overestimate of actual incidence against pre-test probability was 35.9%.
The cells highlighted in blue in table 3 represent low risk patients with a pre-test probability of CAD of 10-29%, according to NICE CG95. In our cohort, only 2.5% (n=7, 95% CI 1.2 – 5.0) of low risk patients were diagnosed with CAD (Table 4). On average, the pre-test probability of CAD exceeded the found incidence of CAD by 18.6% (Table 3).
The white cells in table 3 represent very low risk patients with pre-test probability of CAD <10% according to NICE CG95. In our cohort, only 0.28% (n= 1, 95% CI 0.1 – 1.6) of patients were diagnosed with CAD. Average overestimation in this group was 6.5% in each cell.
ESC guidelines pre test probabilities compared against cohort data
Table 5: A comparison of ESC pre-test probabilities with cohort patient data.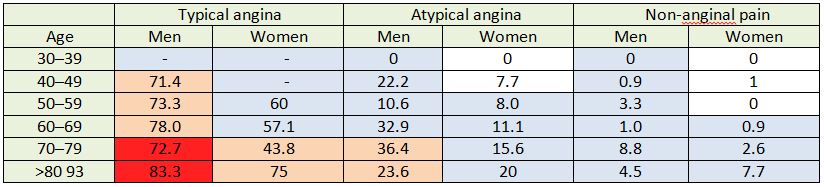
Each cell shows the proportion (%) of cohort patients from each group diagnosed with CAD. Each cell is colour coded to correspond with the ESC estimated pre-test probability. Dark red cells represent >85% probability, pale pink cells represent 66-85% probability, pale blue cells represent 15-65% probability and white cells represent <15% probability.
Table 6: A comparison of ESC pre-test probabilities and cohort patient data
The risk of CAD as predicted by ESC guidelines on the left compared with the actual number of cohort patients in each category and the proportion of those patients diagnosed with significant CAD.
The average discrepancy between pre-test probability of CAD, according to the ESC’s risk stratification table, and actual incidence of CAD in cohort patients was 20.7%. In 28% of cells, the pre-test probability of CAD exceeded the found incidence of CAD by 30% or more (Table 5).
The cells highlighted in dark red in table 5 represent very high risk patients with a pre-test probability of CAD greater than 85%, according to ESC guidelines (Table 5). 73.4% (n= 58, 95% CI 63.7 – 82.7) of cohort patients in this high-risk category were diagnosed with CAD (Table 6). On average, incidence of CAD in each cell has been overestimated by 13% in this category.
The cells highlighted in pale pink in table 5 represent high risk patients, with a pre-test probability of CAD of 66-85%, according to ESC guidelines. 58.5% (n=103, 95% CI 51.1 – 65.5) of cohort patients in this high-medium risk category were diagnosed with CAD (Table 6). On average, the pre-test probability of CAD exceeded the found incidence of CAD in each cell by 17.7% (Table 5).
The cells highlighted in pale blue in table 5 represent medium risk patients with a pre-test probability of CAD of 15-65%, according to ESC guidelines. 6.4% (n=93, CI 5.3 –7.8) of cohort patients in this risk category were diagnosed with CAD (Table 6). On average, the pre-test probability of CAD exceeded the found incidence of CAD by 24.1%in each cell (Table 5).
The cells highlighted in white in table 5 represent patients whose pre-test probability of CAD was less than 15% according to ESC guidelines. Only 0.76% (n=2, 95% CI 0.2 –2.7) of cohort patients in this risk category were diagnosed with CAD (Table 6). On average, pre-test probability of CAD exceeded found incidence of CAD in each cell by 6.2% (Table 5).
Discussion
Only 15% of a total of 1968 patients referred to RACPC were diagnosed with significant CAD. The majority (70%) of referred patients had “non-anginal” chest pain and low pre-test probabilities of CAD, reflecting the importance ascribed by General Practitioners of ruling out ischemic heart disease as the underlying cause for chest pain, even in low risk patients. This may not be surprising given the large media attention to heart disease and sustained campaigns for early warning signs of heart attack in the British media. It is therefore of great public interest for cardiac disease to be identified.
NICE CG95 pre test probabilities compared against cohort data
Comparing cohort data to the pre-test probabilities of CAD outlined in NICE CG95, NICE have overestimated the number of patients likely to have CAD in the majority of groups. Strict adherence to NICE CG95 therefore carries the risk of over-investigating patients. NICE recommend CT calcium scoring as the first line investigation for patients with a low (10-29%) pre-test probability of CAD. 284 patients fall into this category and only 7 patients were shown to have CAD. This means that 40.5 patients need to be treated in order to identify 1 positive patient (NNT= 40.5).
In patients with a medium (30-60%) pre-test probability of CAD, NICE recommends functional imaging as the first line diagnostic investigation. In our cohort 544 patients would undergo functional imaging, but only 24 of these patients would be diagnosed with CAD, NNT=22.7.
Finally, in patient groups with a high (61-90%) pre-test probability of CAD, NICE recommends invasive coronary angiography as the first line diagnostic investigation. In our cohort of 1968 patients, 691 patients had a high pre-test probability of CAD, and 214 had significant coronary artery disease on angiography, NNT= 3.2.
Although invasive coronary angiography is considered the gold standard investigation for diagnosing CAD, and permits simultaneous therapeutic intervention, the procedure is not without risk, particularly in elderly patients and those with renal impairment.11 Furthermore, invasive angiography is expensive and is costed by the East Kent Hospitals University NHS Foundation Trust at £1166.02 per procedure (private correspondence).
NICE CG95 offers no guidance on managing patients who have a <10% pre-test probability of CAD. 347 of our cohort patients fell into this very low risk category and only 1 was diagnosed with CAD. Therefore, NICE CG95, if strictly adhered to, would have missed one diagnosis of CAD in our patient cohort.
ESC pre test probabilities compared against cohort data
ESC guidelines tend to offer more conservative estimates of pre-test probability of CAD compared with NICE guidelines. Using the ESC’s risk stratification table, almost all patients, except those with over 85% pre-test probability and those with less than 15% pre test probability, would be investigated for chest pain. This is due to their claim that non-invasive, image-based diagnostic methods for CAD have typical sensitivities and specificities of around 85%, so that roughly 15% of these investigations could be yielding false results. Hence, due to these inaccuracies, in patients with pre-test probabilities of CAD below 15% or above 85%, ESC state that performing no test at all could provide fewer incorrect diagnoses.9
In our patient cohort, 79 patients had very high (>85%) pre-test probability of CAD, but only 58 patients (73%) were diagnosed with CAD. For this patient risk group, ESC guidelines suggest that further investigation may not be necessary and that a diagnosis of CAD may be assumed. Thus, applying ESC guidelines to our cohort could result in 21 patients being incorrectly diagnosed with stable angina, and more serious causes of chest pain, for example pulmonary emboli or gastric ulceration, may be missed. However, in practice, it is likely that many patients in this very high pre-test probability category would have undergone angiography, because patients who have "severe symptoms" or who are clinically thought to have "high risk coronary anatomy" should be offered an invasive angiography with or without pressure wire studies. The vagueness of the guidelines allows interventionists to interpret this in the clinical context.
In ESC guidelines, invasive coronary angiography is not specifically recommended as a first line investigation for stable angina, regardless of the pre-test probability of CAD. In patients with a high (66-85%) pre-test probability of CAD, ESC guidelines recommend non-invasive functional imaging first line. Of the 176 patients who fell into this category, only 102 (58.0%) patients were ultimately diagnosed with CAD.
In patients with medium (15-65%) pre-test probability of CAD, ESC guidelines advise exercise ECG testing (or non-invasive imaging for ischemia if local expertise is available) as first line diagnostic investigations. Of the 1451 patients which fell into this category, only 93 were diagnosed with CAD, NNT= 15.6. Fortunately, exercise ECG testing would not expose the patient to potentially harmful radiation or medication, but their poor diagnostic power may result in the need for further investigations, despite a negative result.
In patients with low risk of CAD (<15%) ESC guidelines suggest making an assumption that the patient does not have CAD and advocates conducting no further investigations. In our cohort, 263 patients fell into this low risk category, two (0.8%) of which were diagnosed with CAD.
The ESC guidelines appear to have higher specificity than the NICE guidelines, and only two patients would have been missed had ESC guidelines been adhered to, compared to one patient missed if NICE guidance was used. Thus, although highly sensitive, ESC guidelines when applied to our cohort have lower sensitivity than NICE guidelines.
Comparison of number of investigations
Following ESC guidance for our cohort of patients would have resulted in fewer diagnostic invasive angiograms being performed than if NICE guidance had been followed. ESC guidance only recommends invasive angiography if first line, non-invasive investigations generate positive results. Overall, however, ESC guidance would result in a greater number of overall investigations being performed.
In total, NICE advises that all 691 of our high risk cohort patients should undergo invasive angiography as a first line investigation. 544 with medium risk should undergo functional testing first and 24 of these patients (assuming an angiogram would follow a positive result) would go on to have invasive angiography. 284 low risk patients should undergo CT calcium scoring first, of which 7 would go on to have functional imaging and angiography if the above logic is followed. This generates a total of 1557 investigations; 722 angiograms, 551 functional imaging investigations and 284 cardiac CT scans.
In comparison, using ESC guidance, 176 of our high risk patients would have functional imaging investigations, 103 patients with positive results would then undergo invasive angiography. 1451 patients would receive exercise ECGs, of which 93 with positive results would undergo functional imaging and invasive angiography. This generates a total of 1916 investigations; 196 angiograms, 269 functional imaging investigations and 1451 exercise ECGs.
If we assume that stress echocardiograms are used as “functional imaging” we can estimate costs for our cohort when applying each set of guidelines. Costs for each investigation are supplied by East Kent Hospitals University NHS Foundation Trust and are as follows: Outpatient elective coronary angiograms are costed at £1,166.02; stress echocardiograms are costed at £132.30; exercise ECGs at £40.26 and CTs of one area at £102.47 (private correspondence). If we were to apply NICE guidelines to our cohort, £841,866.44 would be spent on angiograms, £72,897.30 would be spent on stress echocardiograms and £29,101.48 on CT scans. This is a total of £943,865.22 on investigations.
If we were to apply ESC guidelines to our cohort, £228,539.92 would be spent on coronary angiograms, £35,588.7 would be spent on stress echocardiography and £58,417.26 would be spent on exercise ECGs. A total of £322,545.88 would be spent on investigations. Overall, this is £621,319.34 cheaper than applying NICE guidelines.
Limitations of study
This study is based on data from a single site and may not be nationally representative. The final diagnosis was made clinically by an experienced interventional cardiologist, which introduces subjectivity and the risk of interpreter bias. Not all patients underwent the gold standard of invasive coronary angiography to demonstrate the presence of CAD. However, all patients were seen and fully assessed by a cardiologist and 97% underwent investigations if deemed necessary.This study has all the limitations of a registry study. In addition, costs for investigations may vary throughout the country, and indeed the world, with varying expertise available.
Conclusion
In conclusion, strict adherence to NICE CG95 over-estimates the pre-test probability of CAD in our local population group. This is consistent with previous studies conducted in South London where there is a larger Afro-Caribbean population, as well as with studies conducted in the North of England.8,9 Adherence to ESC guidelines in place of NICE guidelines may enable a more targeted and cost-effective use of investigations. Strict application of the ESC guidelines to the study cohort would have resulted in investigations costing an estimated £322,545.88, compared to £943,865.22 if NICE guidelines were applied. However, conducting fewer investigations carries greater risk of misdiagnosis, and using ESC guidelines in isolation introduces the possibility of assuming CAD in patients without conducting investigations to confirm this.
It is advisable that local cardiology departments audit their stable chest pain guidelines to ensure that the interpretation of pre-test probabilities is in keeping with the local population. Unfortunately there is no ideal policy and local protocols should reflect the local population.
|
Competing Interests None declared Author Details ANDREW CAI, Department of Cardiology, Kings College Hospital, Denmark Hill, London SE5 9RS, UK. JESSICA BALL, St Thomas' Hospital, Westminster Bridge Rd, London SE1 7EH, UK. ANTONINE PINEAU-MICTHELL, Tunbridge Wells hospital, Tonbridge Road, Tunbridge Wells, Kent TN2 4QJ, UK. KATIE BROWN, Tunbridge Wells hospital, Tonbridge Road, Tunbridge Wells, Kent TN2 4QJ, UK. BNEJAMIN COOPER, Tunbridge Wells hospital, Tonbridge Road, Tunbridge Wells, Kent TN2 4QJ, UK. KUNO BUDACK, Tunbridge Wells hospital, Tonbridge Road, Tunbridge Wells, Kent TN2 4QJ, UK. CORRESPONDENCE: JESSICA BALL, St Thomas' Hospital, Westminster Bridge Rd, London SE1 7EH, UK. Email: jb8511@my.bristol.ac.uk |
References
- Ruigomez A, Rodriguez LA, Wallander MA, et al. Chest pain in general practice: incidence, comorbidity and mortality. Family Practice. 2006;23(2):167-74
- Boden WE, O'Rourke RA, Teo KK, et aland the COURAGE Trial Research Group. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503–1516
- Stergiopoulos K, Boden WE, Hartigan P, et al. Percutaneous Coronary Intervention Outcomes in Patients With Stable Obstructive Coronary Artery Disease and Myocardial Ischemia - A Collaborative Meta-analysis of Contemporary Randomized Clinical Trials JAMA Intern Med. 2014;174(2):232-240
- National institute for health and care excellence. NICE guideline CG95. Chest pain of recent onset: assessment and diagnosis of recent onset chest pain or discomfort of suspected cardiac origin. London: NICE 2010. Available from:http://guidance.nice.org.uk/CG95 (accessed December 2014)
- Diamond GA and Forrester JS. Analysis of probability as an aid in the clinical diagnosis of coronary artery disease. New England Journal of medicine 1979; 300: 1350-8
- Pryor DB, Shaw L, McCants CB, et al. Value of the history and physical in identifying patients at increased risk for coronary artery disease. Annals of Internal Medicine 1993: 118(2): 81–90)
- Khan J, Harrison R, Schnaar C, et al. Do NICE tables overestimate the prevalence of significant CAD? Br J Cardio 2014;21:75
- Cucukcu A, Murra I and Anderson S. What’s the risk? Assessment of patients with stable chest pain Echo Res Pract 2015;2(2):41-48
- Montalescot G, Sechtem U, Achenbach S, et al. European Society of Cardiology. ESC guidelines on the management of stable coronary artery disease. The Task Force on the management of stable coronary artery disease of the European Society of Cardiology European Heart Journal 2013; 34: 2949–3003
- Genders TS,Steyerberg EW, Alkadhi H, et al. A clinical prediction rule for the diagnosis of coronary artery disease: validation, updating, and extension. EurHeart J 2011;32:1316–1330
- Tavakol M, Ashraf S and Brener SJ. Risks and Complications of Coronary Angiography: A comprehensive review. Global journal of health science 2012; 4(1): 65 - 93

The above article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.




