Predictors of in-hospital mortality in Covid-19: a study across two peripheral District General Hospitals in UK
Nil Kamal Samanta, Samik Kumar Bandyopadhyay, Dodiy Herman, Biman Chakraborty, Adrian Marsh, Subramanian Kumaran, Lauren Burnard, Gunalan Gnanaseelan , Saoirse Gibson, Benita Florence & Saibal Ganguly
Cite this article as: BJMP 2021;14(1):a003
|
|
Abstract Aim - The mortality from Coronavirus Disease 2019 (COVID-19) has remained a significant medical challenge. Internationally, patient demographics and pre-existing co-morbidities are significant determinants of mortality from COVID-19. The mortality-risk in a local population is difficult to determine. The objective of our study is to examine the risk posed by epidemiological and demographic variables, and co-morbidities in our local population. Method - A retrospective, observational study was conducted on confirmed COVID-19 patients, identified from the local microbiology database. A search of the electronic patient records was performed to collect demographic details and co-morbidities. Chi-square test and logistic regression analysis of the demographic variables and co-morbidities were utilised to calculate the predictive-risk for in-hospital mortality of adult COVID-19 patients. Results - Final analysis included 263 samples. Univariate logistic regression analysis was performed using age as an independent categorical predictor with two cohorts – those <60 and those ≥60 years old. Age (χ2 =17.12, p<0.001) was found to be an independent predictor of mortality – this was independent of sex (χ2 =1.784, p<0.182). Charlson Comorbidity Index (CCI) score was found to be a significant predictor of adverse outcome. The odds of death for patients with CCI scores 0-4 was less than half (44.8%) of those with CCI scores ≥5 (p=0.005). Patients with no pre-existing medical conditions had a lower mortality-risk (OR=0.181, p=0.022) than those with known medical conditions. Pre-existing renal disease predicted a poor outcome (OR=1.996, p=0.027). The odds of death for the patients coming from their own-home was only 26% of the odds for those from a long-term care-home. Long-term care facility, advanced age (OR=1.058, p <0.001), and long-term oral steroid (OR=3.412, p=0.016) use were all associated with a poor prognosis. Conclusion - People aged ≥60 years, residence in a long-term care-home, pre-existing renal diseases, a high CCI score and long-term oral steroids use were associated with an increased mortality-risk. Keywords: Co-morbidities, Covid-19, Immunomodulator, Mortality.Abbreviations: CCI: Charlson Comorbidity Index; CI: Confidence Interval; CKD: Chronic Kidney Disease; CPR: Cardio-pulmonary resuscitation; OR: Odds Ratio; PRH: Princes Royal Hospital; RAAS: Renin angiotensin aldosterone system; RSH: Royal Shrewsbury Hospital; SaTH: Shrewsbury and Telford Hospitals NHS Trust. |
INTRODUCTION
The most recent outbreak of severe acute respiratory syndrome (SARS) has been caused by coronavirus-2 (SARS-CoV-2) – a new single-strand, positive-sense-RNA beta-coronavirus first reported in 2019 in Wuhan, China. The virus has spread to nearly all countries across the world.1-4
SARS-CoV-2 infection, also known as Coronavirus Disease 2019 (COVID-19), replicates mainly in the upper and lower respiratory tract. The transmission of COVID-19 from symptomatic and asymptomatic patients is usually through respiratory droplets, generated by coughing and sneezing or through contact with contaminated surfaces.4,5 The disease has an incubation period of approximately 5.2 days.6
Most infections are mild and uncomplicated.4 After one week of the onset of disease, 5-10% of patients tend to develop pneumonia, needing hospitalisation.4,6 Some of these patients develop further complications, often leading to death.4,6 The overall case fatality rate is 1.4%, with a noticeably higher rate after the sixth decade of life.4
People aged ≥ 60 years, especially with underlying medical conditions – such as cardiovascular disease, hypertension, diabetes mellitus (DM), chronic respiratory disease, cancer, immunodeficiency, obesity – and those of male-sex, have an increased risk of dying.4,7-12 Risk of severe adverse outcome is also associated with an increased number of associated co-morbidities.10
The impact of active cancer, endocrine disorders, autoimmune inflammatory rheumatic diseases etc. on COVID-19 outcomes has been investigated widely.13-18 Divergent views have emerged regarding the role of renin angiotensin aldosterone system (RAAS) inhibitors, steroids, and immunomodulators in COVID-19 mortality.
The objective of our study was to evaluate the risk posed by epidemiological and demographic variables in our local population. We also sought to analyse the impact of co-morbidities on in-hospital mortality in confirmed COVID-19 patients.
METHODS
Study design:
We conducted a retrospective analysis of demographics characteristics (age and sex) and medical co-morbidities – hypertension, chronic heart failure, ischaemic heart disease, DM, thyroid disorders, asthma, chronic obstructive pulmonary disease (COPD), chronic kidney disease (CKD) (eGFR < 60 mL/min/1.73 m2), chronic liver disease, active malignancy, immunosuppression, post-transplant status, chronic inflammatory arthritis and other rheumatic disorders – in all patients with confirmed COVID-19, who were admitted in two peripheral district general hospitals under a single National Health Service (NHS) trust serving primarily the rural population of western England.
Inclusion and Exclusion Criteria:
To determine COVID-19 status, nose and throat-swab specimens were obtained for real-time reverse transcription polymerase chain reactions (rt-PCR) in all adult (≥18 years) patients, attending one of the two district general hospitals (Royal Shrewsbury Hospital, Shrewsbury; and Princess Royal Hospital, Telford) under Shrewsbury & Telford Hospitals NHS Trust (SaTH) in the period from 1st March to 15th May 2020.
Patients who tested positive (either by N gene and ORF1ab gene positive / ORF1ab gene positive or N gene positive) and required subsequent in-hospital management were included in the study. Patients who were discharged after initial senior review (usually by a consultant physician), or brought in as a cardiac-respiratory arrest, were excluded. Re-admissions to the hospital beyond 48 hours following hospital discharge due to COVID-19 were excluded from the study. Patients diagnosed solely on radiological or clinical findings without a positive rt-PCR test were not included in our study.
We analysed the data based on the index-admission (including failed-discharge: re-admission within 48 hours following hospital discharge). No follow-up data was collected post-hospital discharge of these patients.
Data collection & analysis:
A list of all confirmed COVID-19 patients over a 76-day period was identified from the trust microbiology database. A search of the electronic patient records was completed by four members of our team. Supplementary data was gleaned from existing hospital paper records. Patient demographics, presenting symptoms, associated co-morbidities, medications, admission and discharge dates, intensive therapy unit (ITU) admissions, renal profile, referral source and outcomes were recorded in the specifically designed electronic datasheet.
Study Outcome:
The impact of epidemiological and demographic characteristics, and pre-existing medical conditions on the mortality of confirmed COVID-19 patients requiring in-hospital treatment was analysed.
RESULTS
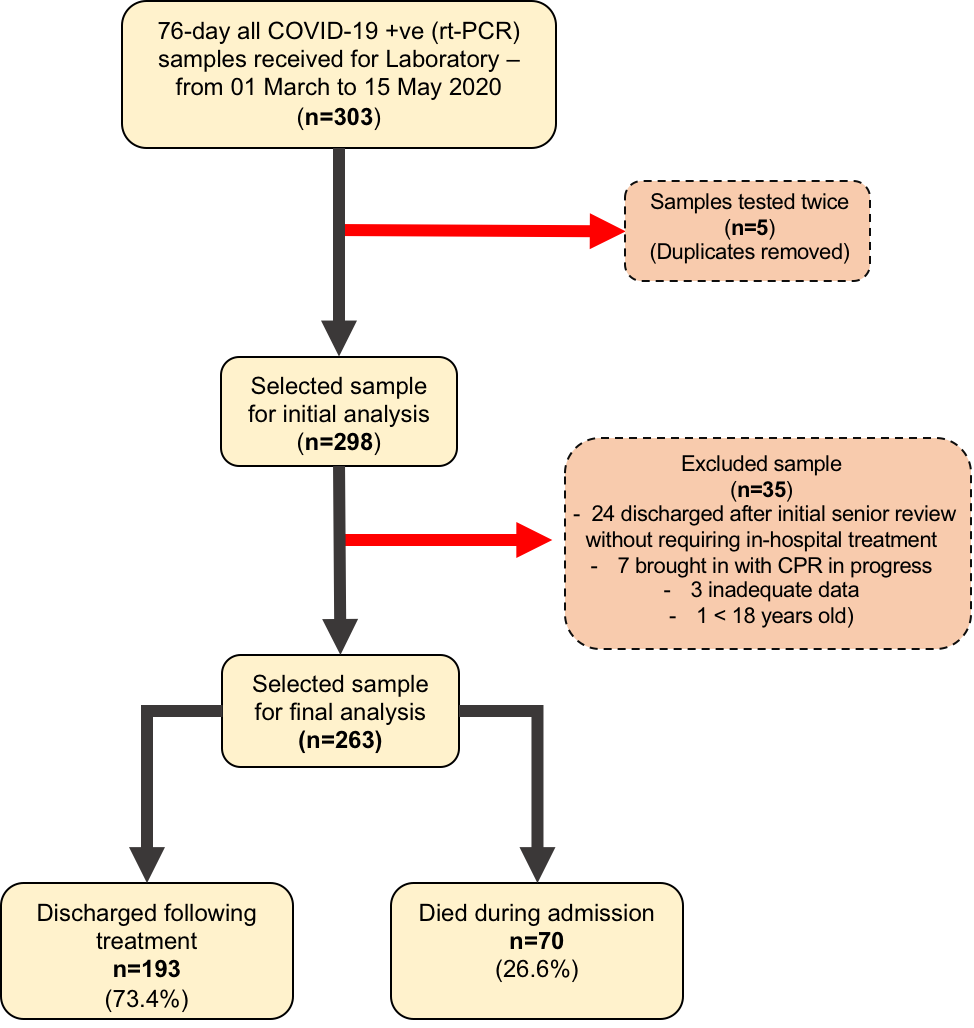
A total of 303 confirmed COVID-19 (rt-PCR positive) samples were collected over a 76-day period. Five patients had been tested twice, and this was accounted for. Thirty-five patients were excluded from the study: twenty-four of them discharged after initial senior review without requiring in-hospital treatment, seven brought in with cardio-pulmonary resuscitation (CPR) in progress, three had inadequate data, and one was <18 years old. Of the 263 patients admitted, 70 (26.6%) died in hospital (Figure-1).
Figure-1: Flowchart of sampling and analysis
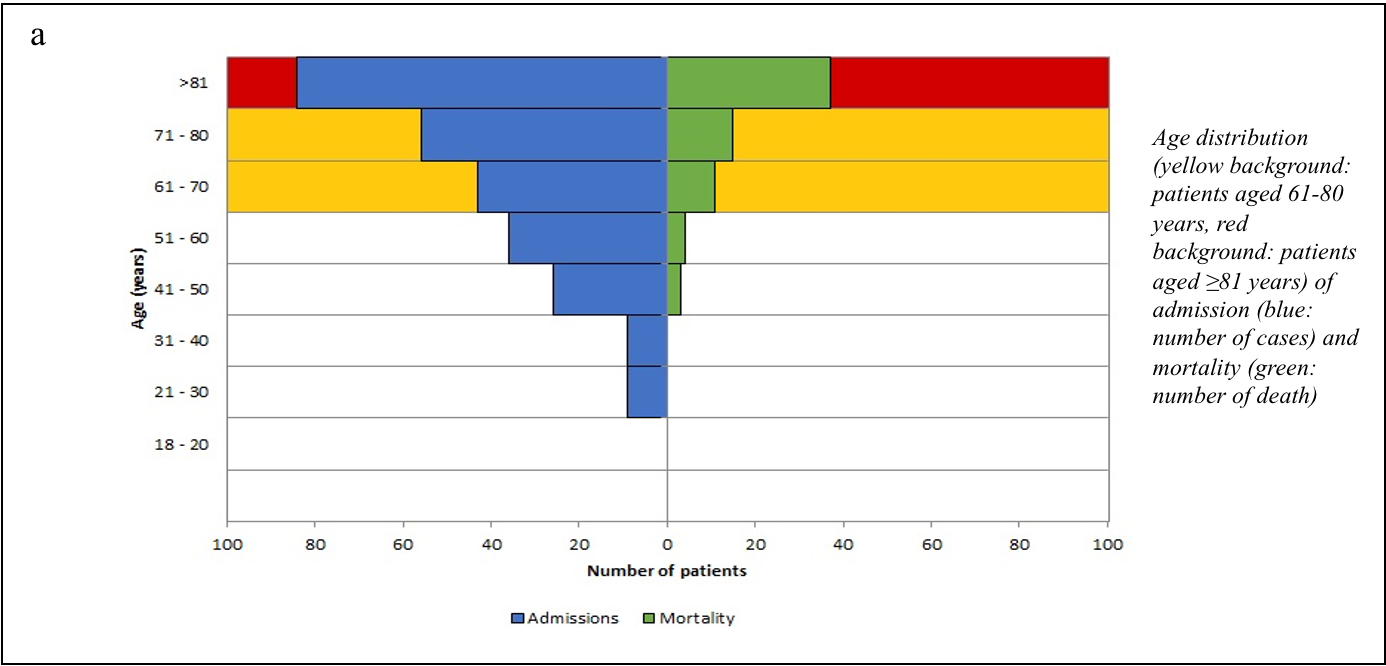
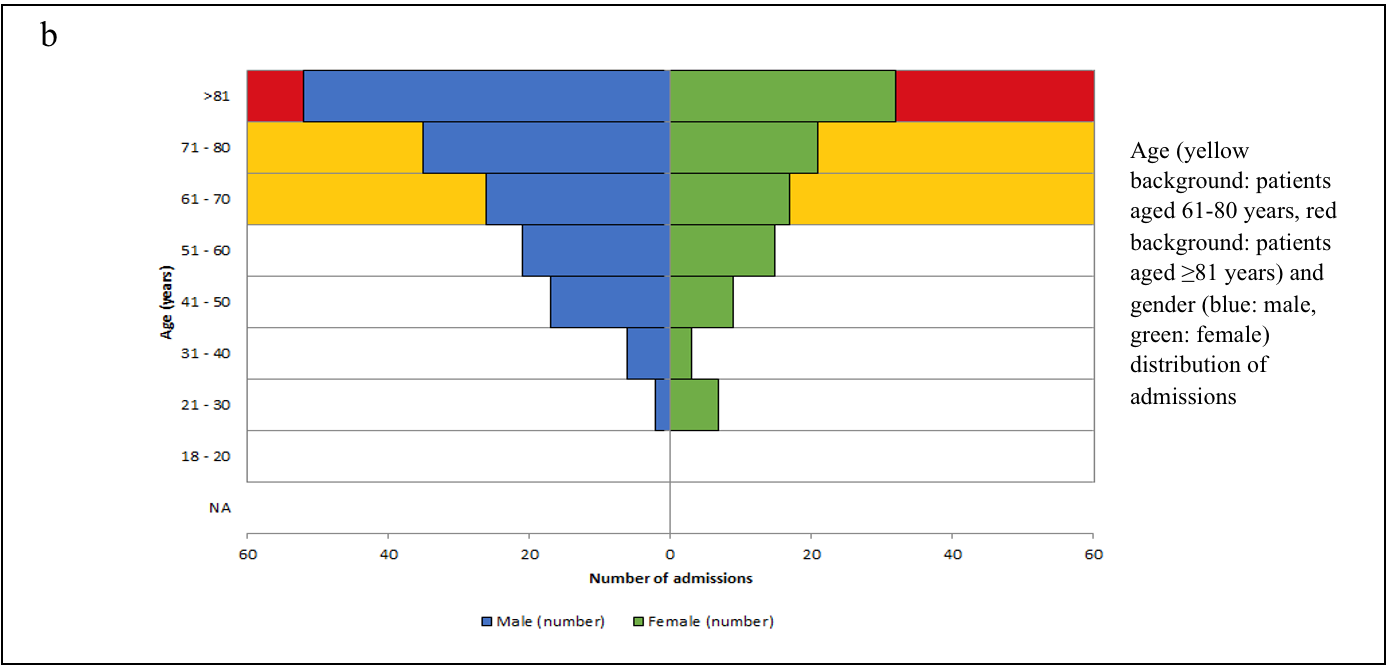
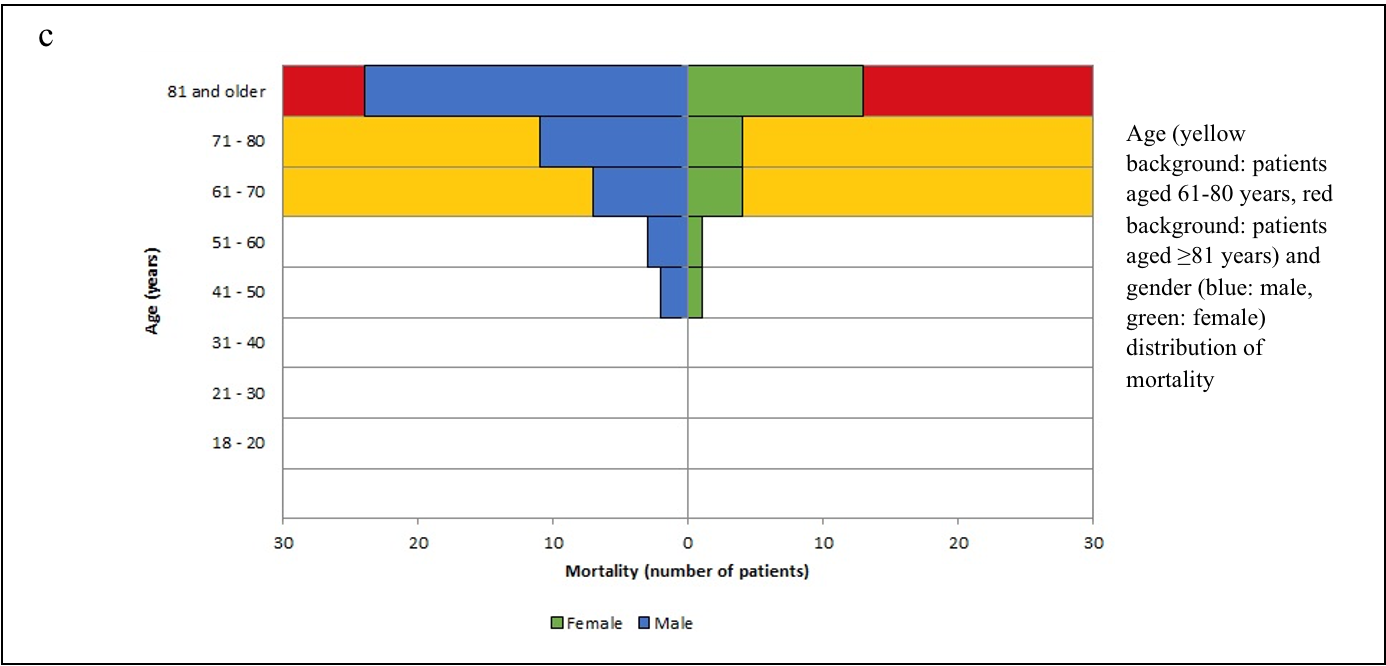
We stratified the mortality rates among the admitted patients by age (Table-1). A chi-square test of independence revealed that the mortality rate was significantly related to an advanced age (χ2 =27.078, p<0.001). The age and sex distributions of admissions and mortality are shown in Figure-2 (a, b, c).
Table-1: Medical admissions and mortality stratified by age
| Age | Admission N(m/f) | Admission
(%) |
Death
N(m/f) |
Mortality
(%) |
Chi-square
(χ2) |
P-value |
| 18 – 20 Years | 0 | 0% | 0 | 0% | 27.078 | <0.001 |
| 21 – 30 Years | 9(2/7) | 3.4% | 0 | 0% | ||
| 31 – 40 Years | 9(6/3) | 3.4% | 0 | 0% | ||
| 41 – 50 Years | 26(17/9) | 9.9% | 3(2/1) | 11.5% | ||
| 51 – 60 Years | 36(21/15) | 13.7% | 4(3/1) | 11.1% | ||
| 61 – 70 Years | 43(26/17) | 16.3% | 11(7/4) | 25.6% | ||
| 71 – 80 Years | 56(35/21) | 21.3% | 15(11/4) | 26.8% | ||
| 81 and Above | 84(52/32) | 31.9% | 37(24/13) | 44.0% | ||
| Total | 263(159/104) | 100.0% | 70(47/23) | 26.6% |
N: number of patients, m: male, f: female.
Figure-2 (a,b,c): Age, Sex, Admission and Mortality pyramids
We considered two age cohorts - below 60 and ≥60 years of age and other relevant demographic parameters (sex and residence in own-home/care-home) to analyse the impact on mortality rates (Table-2). Of the admitted patients, 159 (60.5%) were male, and 104 (39.5%) were female. The mortality rate was strongly associated with advanced age ≥60 years (χ2 =17.120, p<0.001) but independent of sex distribution (χ2 =1.784, p=0.182). However, it was also affected by the care facility (χ2 =18.146, p<0.001) with a higher mortality rate among the group of patients with residence in a long-term care-home.
Table-2: Admission and Mortality stratified by demographic variables
| Variables | Admission (N) | Admission (%) | Death (N) | Mortality (%) | Chi-square
(χ2) |
P-value |
| Age | 17.120 | <0.001 | ||||
| <60 years | 77 | 29.3% | 7 | 9.1% | ||
| ≥60 years | 186 | 70.7% | 63 | 33.9% | ||
| Sex | 1.784 | 0.182 | ||||
| Female | 104 | 39.5% | 23 | 22.1% | ||
| Male | 159 | 60.5% | 47 | 29.6% | ||
| Care facility | 18.146 | <0.001 | ||||
| Own-home | 211 | 80.2% | 44 | 20.9% | ||
| Care-home | 52 | 19.8% | 26 | 50.0% |
N: Number of patients; Care-home: Long-term care in residential or nursing home.
To identify the strength of the associations, we conducted a univariate logistic regression analysis with mortality as the dependent variable and the demography and presence/absence of the co-morbidities as the independent variable (Table-3). We found that age as a continuous predictor had an odds ratio of 1.058 (p<0.001), which translated to increased odds of dying by 5.8% for every year of advanced age. Using age as a categorical predictor with the other two categories, the odds of death for patients aged below 60 years was found to be 0.195 times the odds of death for the patients aged 60 years or above.
Table-3: Univariate logistic regression analysis of the demographic variables and co-morbidities
|
Admission (N) |
Admission (%) |
Died (N) |
Mortality (%) |
Odds Ratio |
95% C.I |
||||
|
Variables |
Categories |
Lower |
Upper |
P-value |
|||||
| Overall | 263 | 100% | 70 | 26.6% | |||||
| Age | 1.058 | 1.035 | 1.082 | <0.001 | |||||
| Age (Cat) | <60 years | 77 | 29.3% | 7 | 9.1% | 0.195 | 0.085 | 0.450 | <0.001 |
| ≥60 Years | 186 | 70.7% | 63 | 33.9% | |||||
| CCI | 1.255 | 1.114 | 1.414 | <0.001 | |||||
| CCI (Cat) | 0 – 4 | 158 | 60.1% | 32 | 20.3% | 0.448 | 0.257 | 0.781 | 0.005 |
| ≥5 | 105 | 39.9% | 38 | 36.2% | |||||
| Sex | Female | 104 | 39.5% | 23 | 22.1% | 0.677 | 0.381 | 1.202 | 0.183 |
| Male | 159 | 60.5% | 47 | 29.6% | |||||
| Care Facility | Own-Home | 211 | 80.2% | 44 | 20.9% | 0.263 | 0.139 | 0.498 | <0.001 |
| Care-Home | 52 | 19.8% | 26 | 50.0% | |||||
| eGFR | 0.961 | 0.950 | 0.973 | <0.001 | |||||
| Active malignancy | Known | 17 | 6.5% | 1 | 5.9% | 0.160 | 0.021 | 1.232 | 0.079 |
| Cardiovascular* disease | Known | 125 | 47.5% | 39 | 31.2% | 1.565 | 0.903 | 2.714 | 0.111 |
| Respiratory§ disease | Known | 55 | 20.9% | 16 | 29.1% | 1.170 | 0.605 | 2.262 | 0.641 |
| DM & endocrine disorders$ | Known | 67 | 25.5% | 21 | 31.3% | 1.370 | 0.745 | 2.518 | 0.312 |
| Renal disease£ | Known | 61 | 23.2% | 23 | 37.7% | 1.996 | 1.082 | 3.681 | 0.027 |
| Rheumatic disorders§§ | Known | 10 | 3.8% | 4 | 40.0% | 1.889 | 0.517 | 6.903 | 0.336 |
| Liver & hepato-biliary diseases± | Known | 8 | 3.0% | 1 | 12.5% | 0.385 | 0.047 | 3.187 | 0.376 |
| Thyroid disorders€ | Known | 16 | 6.1% | 4 | 25.0% | 0.914 | 0.285 | 2.934 | 0.880 |
| Long-term oral Steroids€€ | Known | 17 | 6.5% | 9 | 52.9% | 3.412 | 1.261 | 9.232 | 0.016 |
| Immunomodulators$$ | Known | 9 | 3.4% | 4 | 44.4% | 2.279 | 0.594 | 8.741 | 0.230 |
| No medical condition | Known | 29 | 11.0% | 2 | 6.9% | 0.181 | 0.042 | 0.782 | 0.022 |
*Cardiovascular: Hypertension, chronic heart failure, ischaemic heart disease, chronic arrhythmias; §Respiratory: Asthma, chronic obstructive pulmonary disease, interstitial lung disease, asbestosis; $DM & Endocrine: Diabetes Mellitus (DM), thyroid disorders, Addison's; £Renal: Chronic Kidney Disease (eGFR < 60 mL/min/1.73 m2), on dialysis, chronic nephritis, renal transplant; §§Rheumatic: Rheumatoid arthritis, inflammatory Polyarthritis, chronic vasculitis Syndrome, connective tissue disorders; ±Liver & hepato-biliary: Fatty liver, non-alcoholic steatohepatitis, liver transplant; €Thyroid: Hypothyroidism, hyperthyroidism, post-thyroidectomy; €€Long-term oral steroids: Prednisolone, hydrocortisone, dexamethasone; $$Immunomodulators: Methotrexate, tacrolimus, sirolimus, mycophenolate, dapsone, sulfasalazine and azathioprine.
Based on the Charlson Comorbidity Index (CCI) score, the severity of co-morbidities was categorised into four cohorts: mild/no co-morbidity (CCI:0), moderate (CCI:1-2), severe (CCI:3-4), and very severe (CCI≥5) [Table-4(4a)].
Table-4: Impact of CCI score and specific medical-conditions on admission and mortality
4a) Admission and mortality stratified by CCI score based cohorts
| CCI score | Admission
(N) |
Mortality
(N) |
Mortality
(%) |
OR (95% C.I) | p-value |
| Overall | 263 | 70 | 26.6 | ||
| 0 | 31 | 1 | 3.2 | - | - |
| 1-2 | 59 | 8 | 13.8 | 4.706 (0.56 – 39.49) | 0.154 |
| 3-4 | 68 | 23 | 33.8 | 15.33 (1.97 – 119.67) | 0.009 |
| ≥5 | 105 | 38 | 36.2 | 17.015 (2.23 – 129.78) | 0.006 |
4b) Admission and mortality stratified by specific medical-conditions
| Medical-conditions | Admissions
(N) |
Mortality
(N) |
Mortality
(%) |
OR (95% C.I.) | p-value |
| DM | 54 | 18 | 33.3 | 1.510 (0.791 – 2.883) | 0.212 |
| Thyroid Disorders | 16 | 4 | 25.0% | 0.914 (0.285 – 2.934) | 0.880 |
|
Overall Hypertensives |
75 |
16 |
21.3 |
0.707 (0.374 – 1.338) | 0.287 |
|
ACEi/ARB* antihypertensives |
51 |
11 |
21.6 |
0.760 (0.365 – 1.586) | 0.465 |
|
Non ACEi/ARB§ antihypertensives |
24 |
5 |
20.8 |
0.704 (0.253 – 1.964) | 0.503 |
|
Long-term oral steroids |
17 |
9 |
52.9 |
4.053 (1.091 – 15.063) | 0.037 |
|
Immunomodulators |
9 |
3 |
33.3 |
5.101 (0.659 – 39.460) | 0.119 |
N: Number of patients; DM: Diabetes Mellitus; *RAAS-inhibitors; §Non RAAS-inhibitors.
The impact of CCI score-based cohorts on mortality are shown in Figure-3 (a-f). CCI value also predicted significant association with odds ratio 1.255 (p<0.001). If the CCI score was utilised as a categorical predictor with the other two parameters (age and place of primary care), it remained a significant predictor with the odds of death for the patients with CCI-scores between 0-4 turning out to be 44.8% (p=0.005) of the odds of death for the patients with CCI scores ≥5 (Table-3).
Figure-3(a - f): Pie-chart representing impact of CCI score-based cohorts on mortality
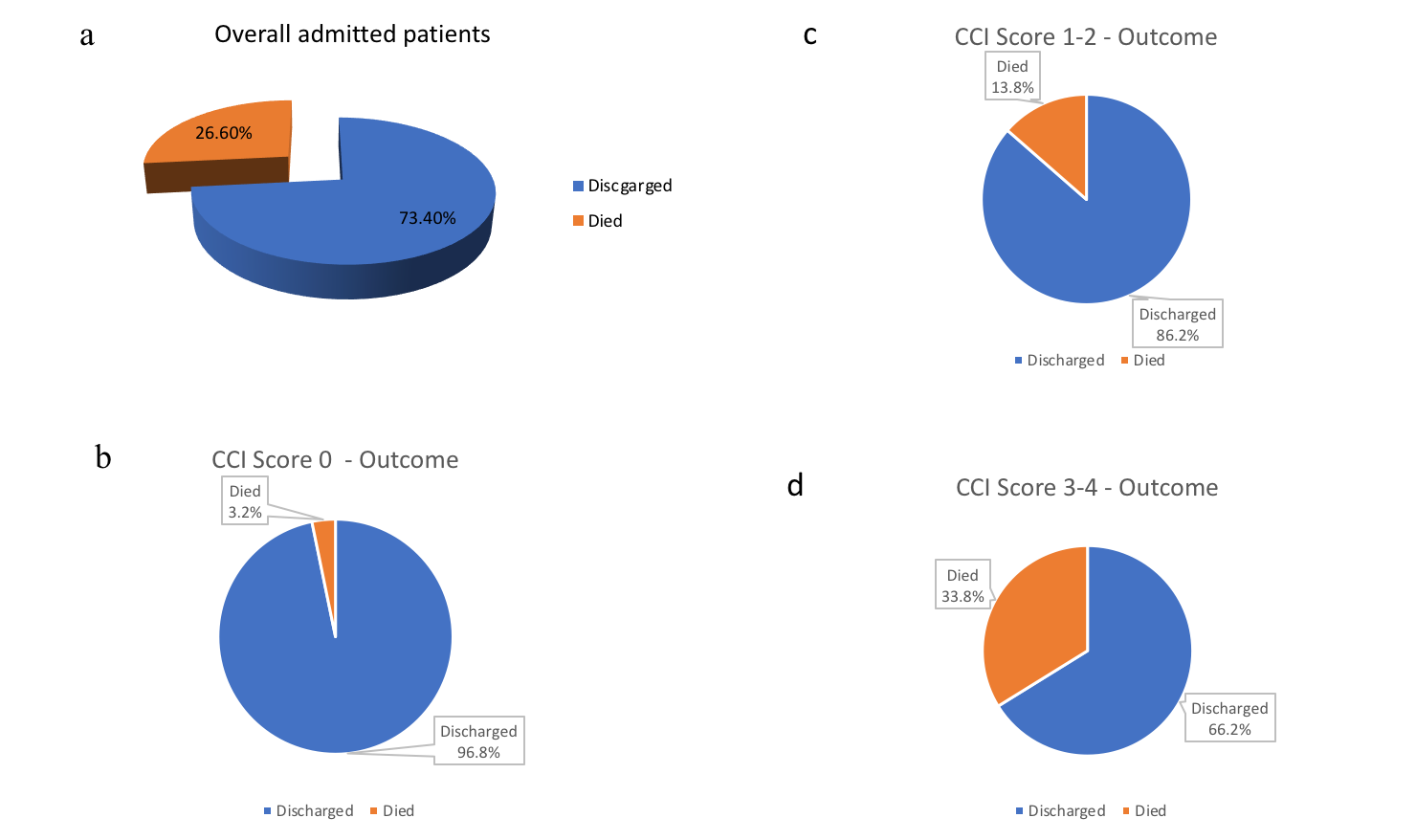
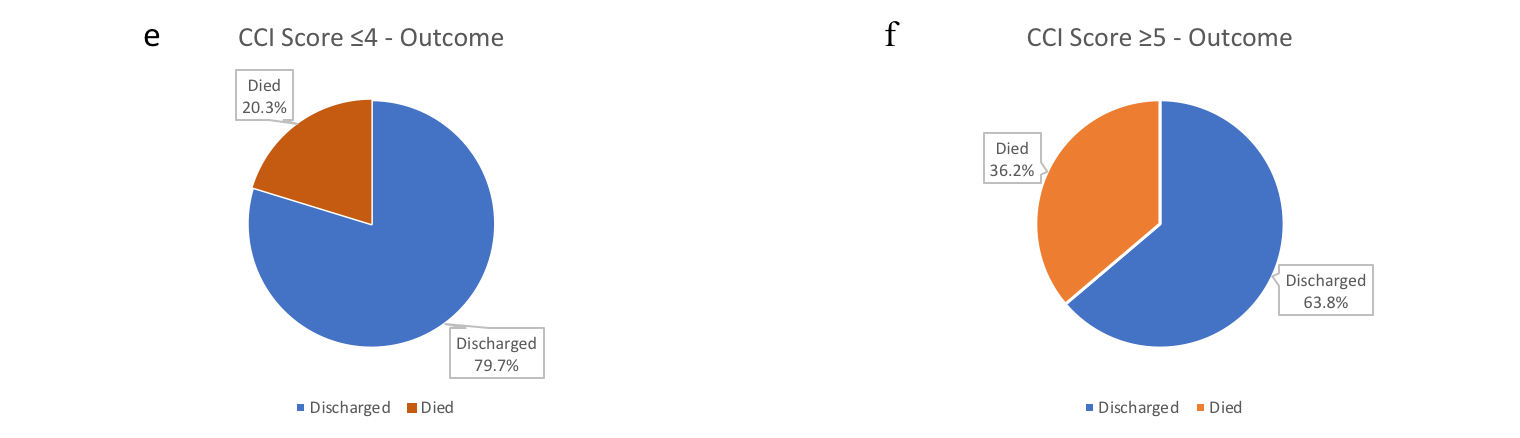
a) Overall admitted patients: discharge and mortality; b) CCI score 0: discharge and mortality; c) CCI score 1-2: discharge and mortality; d) CCI score 3-4: discharge and mortality; e) CCI score ≤4: discharge and mortality; f) CCI score ≥5: discharge and mortality.
Interestingly, the eGFR at presentation turned out to be a significant predictor of mortality (OR=0.961, p<0.001). Of the co-morbidities, pre-existing renal disease was found to be an important predictor of mortality with OR=1.996 (p=0.027). Long-term oral steroids were another significant predictor of mortality, with the odds of death for the patients with long-term oral steroids use being 341.2% (p=0.016) of the odds of death for the patients without such medication. Patients with no background medical conditions (OR=0.181, p=0.022) fared better, with significantly lower odds of death compared to patients with at least one known medical condition (Table-3).
We also analysed the mortality of our patients with specific medical condition-based cohorts [Table-4(4b)]. A high mortality of 52.9% [OR (95%CI): 4.053(1.091–15.063), p=0.037] was observed in patients who were on long-term oral steroids. A 33.3% [OR (95%CI):1.510(0.791–2.883), p=0.212] mortality rate was observed among in-patients with known diabetes on pharmacotherapy.
Many of the demographic variables and the co-morbidities were inter-related – the odds of death for a patient coming from their own-home was only 26% (OR=0.263, p<0.001) of the odds for those residing in a long-term care-home (Table-3). To offset the possibility of any confounding effect, we utilised multiple logistic regression analysis with all the important variables taken together (Table-5). Taking consideration of confounding effects, only age, care facility, presence of active malignancy and long-term oral steroids were found to be significant predictors of mortality. Interestingly, the presence of active malignancy was found to have a lower risk of death – this is possibly due to a bias on account of a relatively small number of patients in that subset of our study. Age was the most significant predictor of mortality, followed by a primary area of the care facility and the presence of active malignancy.
Table-5: Multiple logistic regression analysis of the demographic variables and co-morbidities
|
Odds Ratio |
95% Confidence Interval |
|||
|
Variables |
Lower |
Upper |
P-value |
|
| Age | 1.049 | 1.013 | 1.086 | .007 |
| Sex (Female) | .588 | .296 | 1.165 | .128 |
| Care facility (own-home) | .411 | .195 | .866 | .019 |
| CCI score | 1.051 | .826 | 1.337 | .685 |
| Active malignancy | .078 | .008 | .725 | .025 |
| Cardiovascular disease | .987 | .491 | 1.984 | .971 |
| Respiratory disease | 1.162 | .517 | 2.612 | .716 |
| DM & endocrine disorders | 1.370 | .608 | 3.085 | .448 |
| Renal disease | .901 | .419 | 1.937 | .789 |
| Rheumatic disorders | .927 | .128 | 6.719 | .941 |
| Liver & hepato-biliary diseases | .364 | .030 | 4.357 | .425 |
| Thyroid disorders | .827 | .186 | 3.676 | .803 |
| Long-term oral steroids | 4.053 | 1.091 | 15.063 | .037 |
| Immunomodulators | 5.101 | .659 | 39.460 | .119 |
| No medical condition | .685 | .128 | 3.670 | .658 |
DM: Diabetes Mellitus
DISCUSSION
COVID-19 has taken 800,000 lives world-wide as reported by the World Health Organisation (WHO) on August 30, 2020. A recent systematic review and meta-analysis have reported the association of COVID-19 with a severe disease course in about 23% of infected patients and has a mortality of about 6%.19 The mortality rate varies in different geographical areas. In-hospital mortality was significantly higher in the United States of America (USA) (22.23%) and Europe (22.9%) compared to Asia (12.65%) – (p<0.0001).20 However, there was no significant difference when compared to each other (p=0.49).20 Our study showed a 26.6% in-hospital mortality.
The mean age of the patients in our study was 68.74 years (SD:16.89) – 60.5% of them were male and 39.5% female. 70.7% of these patients were aged ≥60 years. Univariate analysis showed that the mortality rate was significantly age-dependent (OR=1.058, p<0.001) – mortality (33.9%) was higher in patients aged ≥60 years, rising sharply ≥80 years to 44.0% (χ2 =27.078, p<0.001). Our results were consistent with other studies.21
Among the demographic characteristics, mortality-risk was independent of sex distribution (χ2 =1.784, p=0.182) in our study. This is in contrast to a meta-analysis, which reported the association between male-sex and COVID-19 mortality (OR =1.81; 95%CI:1.25–2.62).22 Multicentric studies in the United Kingdom (UK) would be warranted to see the trend in the local population.
Long-term care-home residents suffered 50.0% mortality (χ2 =18.146, p<0.001). The London School of Economics report on May 14, 2020, estimated that the COVID-19 related deaths of care-home residents contributed to 54% of all excess deaths in England and Wales. Our study findings indicate long-term care-homes as hot-spots requiring shielding and protective measures against COVID-19 – a conclusion corroborating other studies.23
We aimed to define the predictive-role of co-morbidities on COVID-19 mortality, an aspect that has been probed earlier as well.7-12 The CCI score remains a reliable method to measure co-morbidity.24 For admission to intensive care, NICE recommended CCI-score ≥ 5 requires critical care advice to help in treatment decision regarding the essential benefit of organ support for seriously unwell COVID-19 patients. We examined the predictive mortality-risk of CCI scores among the admitted patients.
The mortality rate in cohorts with CCI ≤4 and CCI scores ≥5 were 20.3% and 36.2% respectively. The odds of death for CCI ≤4 cohort was less than half (44.8%) compared with CCI scores ≥5 cohort. Based on this finding, we strongly recommend CCI scoring as a clinical risk-stratification tool in COVID-19.
We examined the impact of organ specific co-morbidities on in-hospital mortality in our study as well. Patients with no background medical conditions showed a low mortality rate 6.9% [OR (95%CI): 0.181(0.042–0.782), p=0.022] and had better outcomes with significantly lower odds of death, compared to patients with at least one medical condition on univariate logistic regression analysis (Table-3). The mortality rate was 3.2% in CCI-0 cohort [Table 4(4a)].
The impact of COVID-19 on patients with CKD, glomerulo-nephropathies, on dialysis dependent patients and post renal transplant patients remains unclear. Patients with SARS-CoV-2 infection were frequently found to have renal dysfunction – the latter was associated with greater complications and in-hospital mortality.25 A mortality rate of 3.6%, was reported in patients attending an outpatient haemodialysis centre.26 Another study has concluded 3.07-fold (95%CI:1.43–6.61)mortality among renal failure patients.27 We found, the pre-existing renal disease to be a cause of significant concern with 37.7% mortality [OR(95%CI): 1.996(1.082 – 3.681), p=0.027] with the eGFR at presentation being a significant predictor (OR=0.961, p <0.001) (Table-3).
The use of steroids in COVID-19 continues to be explored.The RECOVERY trial in UK, after evaluation at 28 days, concluded that dexamethasone reduced deaths by one-third in ventilated patients [age-adjusted rate ratio (RR) 0.65; 95% CI: 0.48–0.88; p=0.0003], and by one-fifth in other patients receiving supplemental oxygen with or without non-invasive ventilation (RR 0.80; 95%CI: 0.67 to 0.96; p=0.0021), although no benefit was observed in mild or moderate cases not requiring oxygen support (17.0% vs.13.2%; RR 1.22; 95% CI, 0.93e1.61; p¼0.14). In contrast, a systematic review concluded that the results from retrospective studies are heterogeneous, and it was difficult to assign a definite protective role of corticosteroids in this setting.28 We found long-term oral steroids use to be a significant predictor of mortality – 52.9% [OR(95%CI): 3.412(1.261–9.23), p=0.016] – this was 341.2% of the odds of death for the patients without any long-term oral steroids use (Table-3). The sample size of this cohort was relatively small with 9 deaths out of 17 patients. However, based on our results, it may be safe to suggest that further population-based studies would be required to determine the impact of long-term oral corticosteroid use in COVID-19.
A major proportion of endocrine disorders are of autoimmune aetiology. The impact of thyroid disorders on COVID-19 is yet to be studied widely.15,16 We found no increased risk of mortality [OR (95%CI): 0.914 (0.285–2.934), p=0.880] in patients with thyroid disorders. However, 33.33% [OR(95%CI): 1.510(0.791–2.883), p=0.212] mortality was seen among the diabetic patients on pharmacotherapy in our study [Table-4(4b)].
Pre-existing hypertension is an accepted risk factor for COVID-19 mortality.26,27 However, the role RAAS-inhibitors and upregulation of ACE-2 receptors in COVID-19 mortality call for targeted clinical research for further clarification.29 A meta-analysis of four studies showed that patients treated with RAAS-inhibitors had a lower risk of mortality [RR: 0.65(95%CI:0.45–0.94), P=0.20].30 We did not observe any significant mortality-risk difference between RAAS-inhibitors treatment group [OR(95%CI): 0.760(0.365–1.586), p=0.465] and non RAAS-inhibitor treatment groups [OR(95%CI): 0.704(0.253–1.964), p=0.503] [Table-4(4b)]. We recommend the continuation of RAAS-inhibitors during COVID-19 unless there exist other compelling medical reasons for their discontinuation.
A prospective study in the UK concluded that the mortality from COVID-19 in cancer patients appeared to be driven principally by age, gender, and co-morbidities.13 The study could not identify evidence suggesting cancer patients on cytotoxic chemotherapy, or other anticancer treatment, were at an increased risk of mortality from COVID-19 compared to the general population.13 We also did not detect any increased risk of mortality in patients with active malignancy [OR(95%CI): 0.078(0.008–0.725), p=0.025)] (Table-5).
The impact of various non-specific immunomodulators in COVID-19 outcome remains inconclusive.14 Our study did not reveal any significant predictive mortality-risk with the use of long-term immunomodulators (methotrexate, tacrolimus, sirolimus, mycophenolate, dapsone, sulfasalazine and azathioprine) on multiple logistic regression analysis. We reached the same conclusion with patients suffering from chronic rheumatic disorders on similar analysis (Table-5).
Our study had some unique characteristics. We analysed all the eligible samples over a consecutive 76-day period at the initial peak of the pandemic. The study was conducted across two district general hospitals, allowing an insight into two differently located rural populations. We conducted univariate and multiple logistic regression analysis of the demographic variables and co-morbidities to examine the predictive-risk of contributing factors in COVID-19 mortality. The association between CCI scores and in-hospital mortality was also analysed in detail. We included demographic characteristics such as age, sex and residence in a long-term care-home while factoring in the associations.
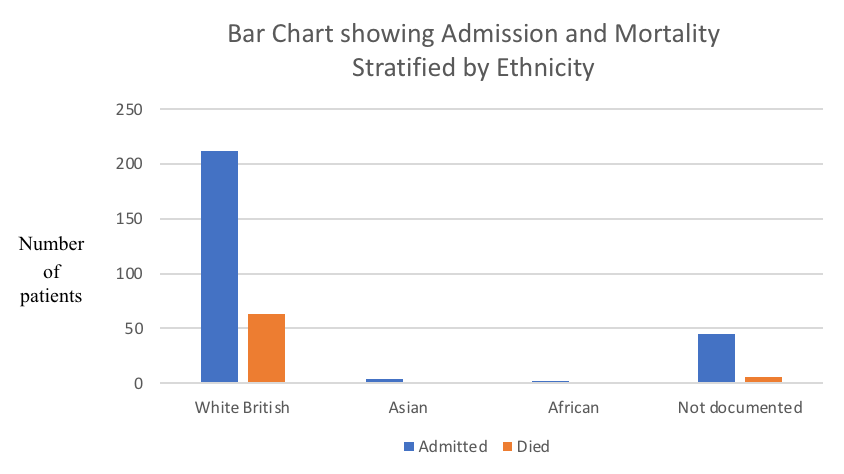
Our study was not without limitations, though. We were unable to study the predictive-risk of obesity, socioeconomic status and ethnicity due to inadequate data. The “White British” group consisted of 80.61% of admitted patients, and no ethnicity was documented in 17.11% of our patients (Table-6, Figure-4).
Table-6: Medical admissions and mortality stratified by ethnicity
| Ethnicity | Admission
(N) |
Admission
(%) |
Died
(N) |
Mortality
(%) |
| White British | 212 | 80.61 | 63 | 29.71 |
| Asian | 4 | 1.52 | 1 | 25.0 |
| African | 2 | 0.76 | 0 | 0.00 |
| Not documented | 45 | 17.11 | 6 | 13.33 |
N: Number of patients
Figure-4: Bar charts showing Admission and Mortality stratified by Ethnicity
We relied solely on electronic database and hospital records to conduct the study retrospectively. The few subsets of patients such as those on prescribed long-term oral steroids, immunomodulators, thyroid disorders, chronic liver disease, and active malignancy had relatively small sample sizes with possible introduction of bias. We did not categorise diabetic patients into insulin dependent/non-insulin dependent or well/poorly glycaemic control cohorts. We did not aim to split the respiratory group into well or poorly controlled asthma or COPD subsets. Patients on a long-term steroid inhalation treatment were not included in the steroid cohort – a more extensive population-based study may be better suited for such an analysis.
CONCLUSIONS
Patients aged ≥ 60 years, residence in a long-term care-home, pre-existing renal disease, multiple co-morbidities (especially those with CCI ≥ 5), and patients on long-term oral steroids need to be considered as having a high risk of dying from COVID-19, along with other established risk factors such as hypertension, diabetes and chronic respiratory disease. RAAS-inhibitors need not be discontinued due to COVID-19. Further studies are necessary to establish links between long-term oral steroids use, chronic rheumatic disease, non-specific immunomodulators and COVID-19 mortality.
|
Acknowledgements Miss Lyndsey Green: Senior BMS, Microbiology, SaTH. Mrs Annette Meredith: ED/AMU Medical Secretary, SaTH. Dr N Zia: Consultant, Emergency medicine, Dudley Group of Hospitals NHS Foundation Trust, Dudley, UK. Dr I Thund: Consultant, Emergency medicine, Dudley Group of Hospitals NHS Foundation Trust, Dudley, UK. Dr S Das: Consultant, Emergency medicine, Stockport NHS Foundation Trust, Stockport, UK. Special thanks to Dr Mohyman El Habishi, Trainee, Anaesthetics, Dudley Group of Hospitals NHS Foundation Trust, Dudley, UK for his contribution of proof-reading, graphics and tables. Competing Interests The authors declare that they have no conflict of interest Author Details NIL KAMAL SAMANTA, MBBS, MRCP, MRCEM, FRCEM, Consultant, Emergency Medicine, Dudley Group of Hospitals NHS Foundation Trust, Dudley, DY1 2HQ, UK. SAMIK KUMAR BANDYOPADHYAY, MBBS, MS, MRCS, FNB(MAS), Speciality Trainee 5, General Surgery, Salford Royal Hospital, Stott Lane, Salford, M6 8HD, UK. DODIY HERMAN, MBChB, FRCEM, Consultant, Emergency Medicine, Shrewsbury and Telford Hospitals NHS Trust, Mytton Oak Road, Shrewsbury, SY3 8XQ. BIMAN CHAKRABORTY, PhD (statistics), School of Mathematics, University of Birmingham, Birmingham B15 2TT, UK. ADRIAN MARSH, MBChB, BSc Med Sci (Hons), FRCEM, Consultant, Emergency Medicine, Shrewsbury and Telford Hospitals NHS Trust, Mytton Oak Road, Shrewsbury, SY3 8XQ. SUBRAMANIAN KUMARAN, MBBS, D Ortho, FECS, FRCEM, Consultant, Emergency Medicine, Shrewsbury and Telford Hospitals NHS Trust, Mytton Oak Road, Shrewsbury, SY3 8XQ. LAUREN BURNARD, MBBS, Speciality Trainee, Acute Common Care Stem, Shrewsbury and Telford Hospitals NHS Trust, Mytton Oak Road, Shrewsbury, SY3 8XQ. GUNALAN GNANASEELAN, MBBS, Foundation Year 2,Shrewsbury and Telford Hospitals NHS Trust, Mytton Oak Road, Shrewsbury, SY3 8XQ. SAOIRSE GIBSON, MBBS, Foundation Year 1, Shrewsbury and Telford Hospitals NHS Trust, Mytton Oak Road, Shrewsbury, SY3 8XQ. BENITA FLORENCE, MBBS, MRCEM, Specialty Doctors and Associate Specialists, Shrewsbury and Telford Hospitals NHS Trust, Mytton Oak Road, Shrewsbury, SY3 8XQ. SAIBAL GANGULY, MBBS, FRCA, FFICM, EDIC, Consultant Anaesthetics and Intensivist, The Royal Wolverhampton NHS Trust, Wolverhampton Road, WV10 0QP, UK. CORRESPONDENCE: Dr Nil Kamal Samanta, Consultant, Emergency Medicine, Dudley Group of Hospitals NHS Foundation Trust, Dudley, DY1 2HQ, UK. Email: Nilkamal.samanta@nhs.net |
References
- World Health Organization. Coronavirus disease 2019 (COVID-19): situation report-37. February 25, 2020. Accessed February 26, 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200226-sitrep-37-covid-19.pdf?sfvrsn=6126c0a4_2
- Wu, C., X. Chen, Y. Cai, et al. Risk Factors Associated with Acute Respiratory Distress Syndrome and Death in Patients with Coronavirus Disease 2019 Pneumonia in Wuhan, JAMA Intern Med. 2020;180(7):934-943.
- Xu X, Chen P, Wang J, et al. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for the risk of human transmission. Sci China Life Sci. 2020;63:457-60.
- Salzberger B., Buder F., Hitzenbichler F., et al. Epidemiologie von SARS-CoV-2-Infektion und COVID-19 Epidemiology of SARS-CoV-2 infection and COVID-19. Der Internist. 2020;61:782-788.
- PHE Guidance: Transmission characteristics and principles of infection prevention and control. Updated June 18, 2020.
- Junejo Y, Ozaslan M, Safdar M, et al. Novel SARS-CoV-2/COVID-19: Origin, pathogenesis, genes and genetic variations, immune responses and phylogenetic analysis. Gene reports. 2020;20:100752. DOI 10.1016/j.genrep.2020.100752
- Srivastava K. Association between COVID-19 and cardiovascular disease. IJC Heart and Vasculature. 2020;29:100583. DOI 10.1016/j.ijcha.2020.100583
- Hafiane A. SARS-CoV-2 and the cardiovascular system. Clinica Chimica Acta. 2020;510:311-316.
- Escalera-Antezana JP, Lizon-Ferrufino NF, Maldonado-Alanoca A, et al. Risk factors for mortality in patients with Coronavirus Disease 2019 (COVID-19) in Bolivia: An analysis of the first 107 confirmed cases. Le infezioni in medicina. 2020;28 (2):238-242.
- Chanyuan Ye, Shanyan Zhang, Xiaoli Zhang , et al. Impact of co-morbidities on patients with COVID‐19: A large retrospective study in Zhejiang, China. J Med Virol. 2020;1-9. https://doi.org/10.1002/jmv.26183
- Li, Xintao, Guan, Bo, Su, Tong, et al. Impact of cardiovascular disease and cardiac injury on in-hospital mortality in patients with COVID-19: a systematic review and meta-analysis. Heart 2020;0:1-6.
- Pranata R; Huang I; Lim MA, et al. Impact of Cerebrovascular and Cardiovascular Diseases on Mortality and Severity of COVID-19 - Systematic Review, Meta-analysis, and Meta-regression. J. Stroke Cerebrovasc. Dis. 2020;29(8):104949 DOI 10.1016/j.jstrokecerebrovasdis.2020.104949
- Lee LYW, Cazier JB, Starkey T, Turnbull CD, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395(10241):1919-1926.
- Rizk JG, Kalantar-Zadeh K, Mehra MR, et al. Pharmaco-Immunomodulatory Therapy in COVID-19. Drugs. 2020; DOI 10.1007/s40265-020-01367-z
- Boelaert K, Visser WE, Taylor PN, et al. ENDOCRINOLOGY IN THE TIME OF COVID-19: Management of hyperthyroidism and hypothyroidism. Eur. J. Endocrinol 2020;183 (1):G33-G39.
- Dworakowska D, Grossman AB. Thyroid disease in the time of COVID-19. Endocrine. 2020;68 (3):471-474.
- Freites Nuñez D.D., Leon L, Mucientes A, et al. Risk factors for hospital admissions related to COVID-19 in patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis. 2020;0:1-7.
- Santos CS, Morales CM, Álvarez ED, et al. Determinants of COVID-19 disease severity in patients with underlying rheumatic disease. Clin. Rheumatol. 2020;39:2789-2796.
- Li J; Huang DQ; Zou B; Yang H; Hui WZ et al. Epidemiology of COVID-19: A Systematic Review and Meta-analysis of Clinical Characteristics, Risk factors and Outcomes. J Med Virol. 2020;1-10.
- Goel. Sunny, Jain. Tarun, Hooda. Amit, et al. Clinical Characteristics and In-Hospital Mortality for COVID-19 Across The Globe. Cardiol Ther. 2020; DOI 10.1007/s40119-020-00189-0
- Mahase E. Covid-19: Death rate is 0.66% and increases with age, study estimates. BMJ. 2020;369:m1327. DOI 10.1136/bmj.m1327
- Ortolan A, Lorenzin M, Felicetti M, et al. Does gender influence clinical expression and disease outcomes in COVID-19? A systematic review and meta-analysis. IJID. 2020;99:496-504.
- Kemenesi. Gábor, Kornya. László, Tóth, Gábor Endre, et al. Nursing homes and the elderly regarding the COVID-19 pandemic: situation report from Hungary. GeroScience. 2020;42:1-7.
- De Groot V, Beckerman H, Lankhorst G.J, et al. How to measure comorbidity: A critical review of available methods. JCE. 2003;56(3):221-229.
- Uribarri Aitor, Núñez-Gil Iván J, Aparisi Alvaro, et al. Impact of renal function on admission in COVID-19 patients: an analysis of the international HOPE COVID-19 (Health Outcome Predictive Evaluation for COVID 19) Registry. J. Nephrol. 2020;33 (4):737-745.
- Farouk SS, Fiaccadori E, Cravedi P, et al. COVID-19 and the kidney: what we think we know so far and what we don't. J. Nephrol. 2020; DOI 10.1007/s40620-020-00789-y.
- Kim Dong Wook, Byeon Kyeong Hyang, Kim Jaiyong, et al. The Correlation of Co-morbidities on the Mortality in Patients with COVID-19: an Observational Study Based on the Korean National Health Insurance Big Data. J Korean Med Sci. 2020;35(26):e243.
- Singh AK, Majumdar S, Singh R; Misra A. Role of corticosteroid in the management of COVID-19: A systemic review and a Clinician's perspective. Diabetes Metab Synd. 2020;14 (5):971-978.
- Hanff TC, Harhay MO, Cohen JB, et al. Is There an Association Between COVID-19 Mortality and the Renin-Angiotensin System? A Call for Epidemiologic Investigations. Clinical infectious diseases. Clin Infect Dis. 2020; 71(15): 870-874.
- Gao C, Cai Y, Zhang K, et al. Association of hypertension and antihypertensive treatment with COVID-19 mortality: a retrospective observational study. Eur. Heart J. 2020;41(22):2058-2066.

The above article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.




