Practical management of aromatase inhibitor-induced bone loss in breast cancer patients
Nilofer Husnoo, Mohummad Shaan Goonoo and Sohail Abbas
Cite this article as: BJMP 2016;9(4):a931
|
|
Abstract Background: Aromatase inhibitors (AIs) are routinely offered to post-menopausal patients with oestrogen receptor-positive early invasive breast cancer (BC). AIs can cause significant bone loss. Several guidelines exist on the management of AI-induced bone loss (AIBL). Aim: To establish practical adherence to guidelines of the assessment and management of AIBL in BC patients Methods: Retrospective audit in a large general practice of patients started on AIs between 2008 and 2015, against national (United Kingdom) guidance and review of the English literature using Ovid Medline and Embase databases Results: 25% (n=3) of patients in our audit did not have a baseline bone mass density (BMD) measurement when an AI was initiated. The mean interval between baseline and repeat BMD measurements was 4.1 years (national recommendation being 2 years). 7 studies assessing practical adherence to guidance were identified in the literature review. The review highlighted suboptimal rates of BMD measurements in BC patients on AIs. Patients diagnosed with osteoporosis do not appear to all be receiving treatment with antiresorptive therapy. Reasons identified for deviation from guidance in large studies include poor awareness of guidelines amongst general practitioners and lack of clarity regarding who the responsibility of bone health management lies with (hospital specialists vs. community team). Conclusion: Guidelines on AIBL have existed for years. We have summarised current evidence on its management, showing that significant gaps in adherence are still present worldwide. Institutional guidelines should be implemented to improve local compliance. Guidelines should also be updated in line with emerging evidence on AIs. Keywords: Aromatase inhibitors; bone loss; breast cancer; hormonal therapyAbbreviations: AI - aromatase inhibitor; AIBL - Aromatase inhibitor induced bone loss; BC - breast cancer; NICE - National Institute of Health and Clinical Excellence; ER - oestrogen receptor |
Introduction
Oestrogen receptors (ERs) are expressed in a large proportion (approximately 70%1) of breast cancers (BCs). Oestrogen stimulates the growth of breast epithelial cells (both normal and cancerous) by binding to these receptors. Aromatase inhibitors (AIs) prevent the conversion of androstenedione to oestrogen by the enzyme aromatase in peripheral tissues, which is the predominant source of oestrogen in post-menopausal women. Consequently, they are routinely offered to post-menopausal women with ER-positive early invasive breast cancer as adjuvant therapy2. However, decreased residual oestrogen levels are associated with increased bone resorption by osteoclasts. The menopause initiates an accelerated phase of bone loss lasting 4 to 8 years, which is followed by a slower phase which continues indefinitely3. AI-induced bone loss (AIBL) occurs at a higher rate than natural menopausal bone loss4. Women are therefore at increased risk of fractures while they are on AI therapy5, with an associated higher rate of fractures as demonstrated in the ATAC trial6.
Recent data have supported more prolonged use of AIs (10 years instead of 5) to achieve lower BC recurrence rates7. This may lead to changes in future clinical practice in that ER-positive BC patients may be on an even longer course of AIs. This is likely to translate into a higher fracture risk in patients on long term treatment, and bone health in these patients should remain an important consideration.
Several guidelines have emerged over the years, as summarised by Hadji et al8, to aid the assessment of fracture risk in women receiving BC treatment, and management of AIBL. In the UK, the guidance in use and recommended by the National Institute of Health and Clinical Excellence (NICE) is a UK expert group consensus position statement issued in 2008 (Guidance for the Management of Breast Cancer Treatment-Induced Bone Loss)9. This includes two treatment algorithms for the assessment and management of bone loss in early BC: one for women with adjuvant treatment-induced premature menopause and the other for postmenopausal women starting adjuvant AI.
Despite the existence of various guidelines on the management of AIBL in BC patients, few articles have been published on the practical adherence to guidance. We carried out an audit of the management of AIBL in BC patients in a large general practice (with roughly 9000 registered patients) in Bradford (UK). Given the small number of eligible patients in our study, we undertook a review to identify all studies in the English literature assessing practical adherence to guidance on AIBL to establish whether gaps identified in our practice reflects a more widespread issue.
Our study
Methods
We carried out a retrospective study in a general practice in April 2015. Using the clinical electronic system used at the practice (SystemOne), we performed a search for all registered patients documented to currently be on AIs or to have previously been on them at any point, for the treatment of BC, using the search terms “anastrazole”, “Arimidex”, “exemestane”, “Aromasin”, “letrozole” and “Femara”. We excluded male patients (not addressed by current guidelines) and patients who started their treatment with AIs prior to the issuance of the UK guidance in 2008. For each patient we gathered data on the indication of treatment, menopause status, the date of initiation +/- completion of treatment, details of dual energy Xray absorptiometry (DEXA) scan and bone mass density (BMD), blood biochemistry results, documented risk factors for fractures and details of bone protection treatment. We audited our practice against the UK guidance.
Summary of the UK guidance
All post-menopausal patients starting AIs should have a baseline DEXA within 6 months of treatment initiation. Patients are stratified as low, medium and high risk for fractures based on the baseline T-scores. Medium and high risk patients should have vitamin D and calcium supplements, and high risk patients should be started bisphosphonates. A repeat DEXA scan should be performed 2 years later for medium and high risk patients to re-assess BMD and augment bone protection therapy as appropriate. Patients aged 75 years and above with at least one clinical risk factor for fractures should be started on a bisphosphonate regardless of their baseline BMD.
Results
There were 12 female patients who started AIs for BC treatment from 2008 onwards. Treatment was initiated between the years 2008 and 2014 (inclusive). The mean age was 67 years (range 57-81 years) and all 12 were post-menopausal at the time of adjuvant hormonal therapy initiation. Three were initially on tamoxifen and switched to an AI after 2 years of tamoxifen therapy.
Three patients (25%) did not receive an initial DEXA scan and had no subsequent risk fracture management. One of them was 75 years of age at the time of AI initiation and was on long term steroids (i.e. should have been on a bisphosphonate regardless of BMD), but she was not on a bisphosphonate.
Of the remaining 9 (75%) who did have a DEXA scan,
One was at high risk (T-score -2.7), and was appropriately started on a bisphosphonate and calcium and vitamin D supplements.
7 patients were at medium risk of osteoporotic fractures (T-score range -2.0 to -0.1). All were started on calcium and vitamin D supplements.
7 were eligible to have had a repeat DEXA scan at the time of the study but only 4 had a scan. Of these four, one was found to have incurred significant bone loss and was started on a bisphosphonate.
The mean interval between AI initiation and baseline DEXA was 1.9 months (range 0.2-4.4). The mean interval between the initial and repeat DEXA scans was 4.1 years (range 2.5-5.1).
Figure 1 illustrates the proportion of scans requested by different clinical teams involved in the patients’ care.
Figure 1: Who requests DEXA scans?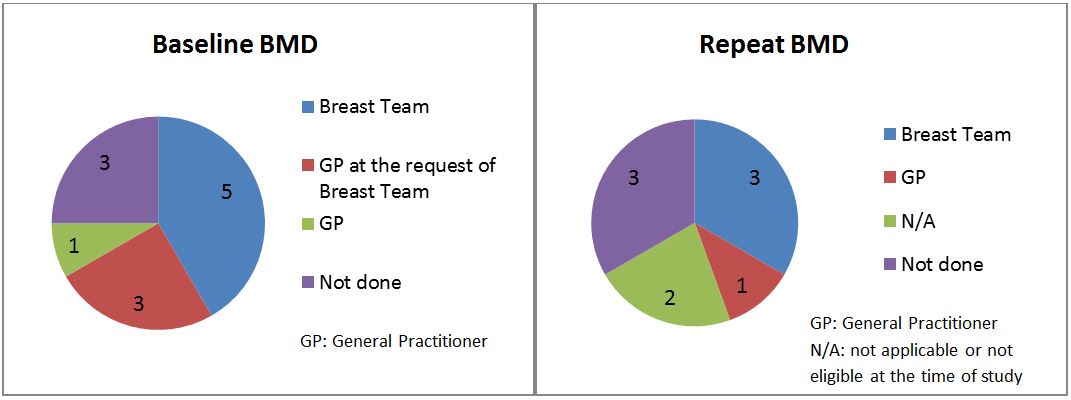
Literature review
Methods
We performed a search with the following terms on the Ovid Medline and Embase databases: “bone loss”, “osteoporosis”, “osteopenia”, “aromatase inhibitor”, “breast cancer”, “guidelines” and “guidance”. Of the 137 results returned after deduplication, we selected original and review articles assessing management of AIBL against established guidelines. We retrieved further papers by reviewing the references of these articles.
Results
The original articles generated are shown in Table 1. While conference abstracts have not been included here, they have been reviewed for the purpose of our discussion.
Table 1: Original articles publishing the results of audits of bone health management in BC patients on AIs against established guidelines
| Authors | Place of study | Guidelines used to define audit standards | Sample size | Adjuvant therapy |
| Roberts R et al10 | Australia | ASCO*, ESMO*, Hadji et al8, Belgian Bone Club | 42 | Both AI and tamoxifen |
| Spangler L et al11 | Washington, USA | ASCO* | 342 | AI |
| Bosco D12 | Italy | Results from the ARBI* trial13 | 39 | AI |
| Gibson K et al14 | Colorado, USA | ASCO* | 54 | AI |
| Ligibel et al15 | USA | ASCO*, NCCN*, Hadji et al8 | 9138 | AI |
| Dong et al16 | UK | NICE guidelines based on UK expert group consensus9 | 100 | AI |
| Zekri J et al17 | Saudi Arabia | NICE guidelines based on UK expert group consensus9 | 367 | AI |
*ASCO: American Society of Clinical Oncology, ESMO: European Society for Medical Oncology, NCCN: National Comprehensive Cancer Network, ARBI: Arimidex Bone Mass Index and Oral Bisphosphonates
Discussion
The results of our audit show that we are failing to meet our current national standards pertaining to management of AIBL in BC patients. Our literature review confirms that this is a widespread issue and that results from larger studies are in agreement with ours.
25% of our patients never had a baseline BMD measurement. Similar findings have been reported in the literature11,12,14. However, Roberts et al report much higher rates of DEXA screening pre –AI10. Reasons for this were felt to be the presence of an institutional treatment algorithm as well as a survivorship programme.
We had a poor rate of repeat DEXA scans. Gibson et al and Spangler et al also noted that the highest rate of DEXA scanning was around the time of AI initiation compared to after initiation of therapy11,14. For the patients who had a repeat BMD measurement in our study, practice was not in line with recommendations as the interval between the initial and repeat DEXA scans (mean 4.1 years) was much longer than the recommended 2 years. This may be because recommendations made by the breast surgery team were different (intervals of 3 to 5 years being recommended in some cases in clinic letters written to the GP by the breast team).
Gibson et al found that 75% of their patients were on calcium and vitamin D, which deviates from the ASCO guidelines that they audited their results against14. The ASCO guidelines recommend that all BC patients should be on calcium and vitamin D therapy. In some studies10.12 not all women diagnosed with osteoporosis were started on bisphosphonates. Although women diagnosed with osteoporosis were started on bisphosphonates in our cohort, the suboptimal uptake of DEXA scans means that we may have missed the diagnosis in a number of patients.
From the articles included in our literature review, several reasons have been suggested as causes for deviation from guidelines when it comes to management of AIBL in BC patients. Lack of awareness of guidelines, especially amongst general practitioners (GPs), has been recognised as a barrier, as well as the expectation that other healthcare professionals should be addressing this aspect of care10. In our study, DEXA scans were mostly requested by the specialist breast team initiating AIs, or by the GP at the request of the breast team. Based on our experience, it is not clear who the responsibility of bone health management lies with – the breast surgery team, the oncologist or the GP. In a survey of 307 UK-based breast surgeons and oncologists 57% of responders felt that oncologists should be responsible for this18. In practice, patients may be discharged from specialist clinic follow-ups while they are still on hormonal therapy and GPs would be expected to continue their care. When this happened in our cohort of patients, there was no evidence of clear written communication from specialist teams to the GP regarding outstanding aspects of care that the GP would be expected to follow up.
An analysis of five different guidelines regarding antiresorptive treatment in postmenopausal women with hormone-receptor positive BC showed that little consistency exists among the five guidelines19. The variety of guidelines and recommendations regarding bone loss in BC patients probably leads to inconsistency in practice. In our study, specialist teams have sometimes recommended an interval of 3 to 5 years between BMD tests, deviating from the national recommendation of 2 years. This can translate into confusion when care is taken over by the community team after the patient is discharged from the specialist team.
Recommendations
We therefore suggest that institutional guidelines on bone health management in BC patients on AIs (as well as other hormonal therapies) should be created to improve awareness amongst clinicians as this has shown to improve rates of DEXA scanning10. Local guidelines should closely mirror national guidelines to allow delivery of standardised care across the country, but should include clear recommendations as to which local team should be responsible for bone health management, as well as recommendations regarding the creation of a care plan for general practitioners when the patient is discharged from specialist teams.
A UK-based study has shown that a “one stop” nurse-led bone health clinic within the breast care service can be a cost-effective way of ensuring adherence to guidelines20. Patients to be started on an AI are identified by the multidisciplinary team (MDT). They are referred to the clinic which arranges a baseline DEXA and other appropriate investigations. Such a clinic may be a consideration in institutions where resources allow. Studies have also shown that simple interventions such as presentations at MDT meetings and display of posters to increase awareness of guidelines amongst clinicians have led to significant improvement in compliance16,17.
Lack of patient awareness of the negative effects of AIs has also been highlighted in the literature21. Improving patient education can improve patients’ compliance with treatment and decrease the rates of unattended appointments for BMD screening. It can also give more control to patients over management of their bone health, as they may be able to discuss with their clinicians where they notice a gap (e.g. if they have failed to receive an appointment for a DEXA scan). Ligibel et al have noted that women from areas with lower levels of education are less likely to undergo BMD tests15.Patient education can also help reduce the impact that such health-seeking behaviours have on compliance to bone health management.
Current guidelines make no mention of bone health management in male BC patients on hormonal therapy. Although they constitute a small percentage of BC patients, it would be reasonable to include recommendations of their bone loss management in updated guidelines so that this aspect of their care is not neglected.
Strengths and limitations
Our audit is limited by its small sample size and its retrospective nature which meant that we relied on documentation of variable accuracy. We had no information regarding patients who failed to attend appointments despite their clinicians’ invitations for DEXA scans or biochemistry tests, and no information on compliance to medication. However, the results from recent conference abstracts on UK based studies22,23 generated from our literature review reflect our results, suggesting that this is indeed a national issue. The literature review presented is the most extensive currently available on the subject, gathering up-to-date evidence on worldwide compliance to guidelines on AIBL.
Conclusion
Although the sample size of our study does not allow us to draw conclusions purely based on our data, the literature review that it has prompted has shown that several years after issuance of various guidelines on the management of BC treatment-induced bone loss, in particular AIBL, important gaps still exist in practice. We have presented a summary of up-to-date evidence in the literature to identify potential reasons for this and possible solutions to the current problems, hoping that this will improve current practice.
However, the current guidelines are now several years old. In the last few years, there has been a lot of research on the role of bisphosphonates in BC. A consensus paper assessing recent evidence has suggested that bisphosphonates should be considered for the prevention of bone loss in patients with a T score of <-2.0 or with at least two clinical risk factors for fracture24. The paper also suggests considering the use of bisphosphonates as adjuvant BC treatment, based on a large meta-analysis including 18 766 patients which demonstrated significant benefits of bisphosphonates in terms of prevention of bone metastases and BC survival in postmenopausal women25. This may well change routine adjuvant treatment of BC in the next few years and must be taken into consideration if and when new guidelines on the management of AIBL are issued, or when writing local guidelines.
|
Competing Interests None declared Author Details NILOFER HUSNOO,MBBS, MRCS, Bradford Teaching Hospitals NHS Trust, Bradford Royal Infirmary, Duckworth Lane, Bradford, United Kingdom, BD9 6RJ. MOHUMMAD SHAAN GOONOO, Sheffield Teaching Hospitals NHS Trust, Royal Hallamshire Hospital, Glossop Road, Sheffield, United Kingdom, S10 2JF. SOHAIL ABBAS, Kensington Partnership, Kensington Street Health Centre, Whitefield Place, Bradford, United Kingdom, BD8 9LB. CORRESPONDENCE: NILOFER HUSNOO,MBBS, MRCS, Bradford Teaching Hospitals NHS Trust, Bradford Royal Infirmary, Duckworth Lane, Bradford, United Kingdom, BD9 6RJ. Email: niloferhusnoo@doctors.org.uk |
References
- Lumachi F, Brunello A, Maruzzo M, Basso U, Basso SM. Treatment of estrogen receptor-positive breast cancer. Curr Med Chem. 2013;20(5):596-604
- National Institute for Health and Care Excellence. 2009. Early and locally advanced breast cancer: diagnosis and treatment. [ONLINE]. Available at https://www.nice.org.uk/guidance/cg80. [Accessed 28/8/16]
- B. Lawrence Riggs, Sundeep Khosla, and L. Joseph Melton, III. Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002 Jun;23(3):279-302
- Hadji P. Aromatase inhibitor-associated bone loss in breast cancer patients is distinct from postmenopausal osteoporosis. Crit Rev Oncol Hematol. 2009 Jan;69(1):73-82
- Eastell R, Adams JE, Coleman RE, et al. Effect of anastrozole on bone mineral density: 5-year results from the anastrozole, tamoxifen, alone or in combination trial 18233230. J Clin Oncol 2008;26:1051-1057
- The ATAC Trialists' Group. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol 2008;9:45-53
- Goss PE, Ingle JN, Pritchard KI, et al. Extending Aromatase-Inhibitor Adjuvant Therapy to 10 Years. N Engl J Med. 2016 Jul 21;375(3):209-19
- Hadji P, Aapro MS, Body JJ, et al. Management of aromatase inhibitor-associated bone loss in postmenopausal women with breast cancer:practical guidance for prevention and treatment. Ann Oncol. 2011 Dec;22(12):2546-55
- Reid DM, Doughty J, Eastell R et al. Guidance for the Management of Breast Cancer Treatment-Induced Bone Loss: a consensus position statement from a UK expert group. Cancer Treat Rev 2008; 34 (Suppl 1); S3-S18
- Roberts R, Miller M, O'Callaghan M, Koczwara B. Bone health management of Australian breast cancer survivors receiving hormonal therapy. Intern Med J. 2015 Nov;45(11):1182-5
- Spangler L, Yu O, Loggers E, Boudreau DM. Bone Mineral Density Screening Among Women with a History of Breast Cancer Treated with Aromatase Inhibitors. J Womens Health (Larchmt). 2013 Feb; 22(2): 132–140
- Bosco D. Osteoporosis and aromatase inhibitors: experience and future prospects. Clin Cases Miner Bone Metab. 2012 May-Aug; 9(2): 89–91
- Markopoulos C, Tzoracoleftherakis E, Polychronis A, et al. Management of anastrozole-induced bone loss in breast cancer patients with oral risedronate: results from the ARBI prospective clinical trial. Breast Cancer Res. 2010; 12(2): R24
- Gibson K, O’Bryant CL. Screening and management of osteoporosis in breast cancer patients on aromatase inhibitors. J Oncol Pharm Practice (2008) 14: 139-145
- Ligibel JA, O’Malley AJ, Fisher M, et al. Patterns of Bone Density Evaluation in a Community Population Treated with Aromatase Inhibitors. Breast Cancer Res Treat. 2012 Aug; 134(3): 1305–1313
- Dong H, Dayananda P, Preece S, Carmichael Amtul. Are patients with newly diagnosed breast cancer getting appropriate DEXA scans? A District General Hospital experience. BMJ Qual Improv Rep. 2015; 4(1)
- J. Zekri, K. Farag, Assessment of bone health in breast cancer patients starting adjuvant aromatase inhibitors: A quality improvement clinical audit, Journal of Bone Oncology (2016), http://dx.doi.org/10.1016/j.jbo.2016.05.007i
- Lester JE, Dodwell D, Horsman JM, Mori S and Coleman RE. Current management of treatment-induced bone loss in women with breast cancer treated in the United Kingdom. Br J Cancer. 2006 Jan 16; 94(1): 30–35.
- Hadji P, Hartenfels M, Kyvernitakis J, et al. Recommendations for antiresorptive therapy in postmenopausal patients with breast cancer: Marburg AIBL Guideline Evaluation Study (MAGES). Breast Cancer Res Treat. 2012 Jun;133(3):1089-96
- Carr WM. Avoiding the breast cancer and bone health care gap. Conference: Osteoporosis Conference 2014. Birmingham United Kingdom. 2014 Nov 30 – 2014 Dec 02. Osteoporosis International. 2014Nov; 25 (6 SUPPL. 1): S702-S703
- Tham YL, Sexton K, Weiss HL, et al. The adherence to practice guidelines in the assessment of bone health in women with chemotherapy-induced menopause. J Support Oncol. 2006 Jun;4(6):295-8, 304.
- Sibbering M., Ives-Smith K., Rogers V. Clinical audit of the assessment of osteoporosis by axial DXA scanning and treatment/prevention of bone loss in post menopausal women, commenced on adjuvant treatment for breast cancer with aromatase inhibitors. Conference: Association of Breast Surgery Conference and AGM, ABS 2015. Bournemouth United Kingdom. 2015 Jun 15 - 2015 Jun 16. Eur Joun of Surg Onc. 2015 Jun; 41 (6): S27
- Critchley A., Robinson A., El-Asir L., Clark K. Are we doing enough to protect the bones of patients on aromatase inhibitors?. Conference: Association of Breast Surgery Conference and AGM, ABS 2013. Petersfield, Manchester United Kingdom. 2013 May 2 - 2013 May 22. EmbaseEuropean Journal of Surgical Oncology. 2013 May; 39 (5): 478
- Hadji P, Coleman RE, Wilson C, et al. Adjuvant bisphosphonates in early breast cancer: consensus guidance for clinical practice from a European Panel. Ann Oncol. 2016 Mar;27(3):379-90
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG), Coleman R, Powles T, et al. Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet. 2015 Oct 3;386(10001):1353-61.

The above article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.




