The Lost Mirena: What Investigations Are Required ? An Intraperitoneal Levonorgestrel-Releasing Intrauterine System Following Uterine Perforation: Case Report
Shambhu S and Pappas M
Cite this article as: BJMP 2009:2(1) 38-40
|
The Mirena intrauterine system (IUS) has been licensed as a contraceptive in the UK since May 1995. Recent National Statistics suggest the Mirena IUS is used by only 1% of women aged 1649 years who are currently using contraception.1 The Mirena IUS now also has a licence for the management of idiopathic menorrhagia2 and may therefore be used by women who do not require contraception. Uterine perforation is a serious, albeit rare, potential complication of intrauterine device or system use. Women may be informed that uterine perforation occurs in fewer than 1 in 1000 of either copper intrauterine device (IUD) or IUS insertions.3,4 Rate of perforation reported with the Mirena IUS in a large observational cohort study was 0.9 per 1000 insertions.5 In this case report, an intraperitoneal Mirena IUS was detected nearly 4 years after its insertion and perforation of the uterus was diagnosed, despite vaginal hysterectomy and admissions to hospital. This case report demonstrates clearly that whenever there is suspicion from ultrasound scan report of an empty uterus that the IUS has fallen out, and in the persistence of symptoms, we should consider performing an abdominal X-Ray which is an easy, cheap method, to identify the IUS outside the uterus.
Case report
A 33-year-old woman, para 2, with a long standing history of menorrhagia, dysmenorrhoea and tiredness was referred by her GP to the hospital (2002). At the time she was treated for anaemia and felt tiredness. Also she was suffering from dysmenorrhoea; her periods had been regular although in the previous few months she was bleeding PV continuously. Her periods had become heavy after sterilisation (1996). She was anaemic. Cervical cytology had always been normal. In the past she had undergone a laparoscopy for pelvic pain for suspected endometriosis (1997), an appendicectomy (1997) and she was diagnosed with duodenal ulcer (1996). For management of her menorrhagia, she opted for Microwave Endometrial Ablation which was done in August 2002. After that she had an ultrasound scan for erratic bleeding which showed irregular endometrium. The patient was booked for hysteroscopy under general anaesthesia. The procedure was attempted in September 2003 and was abandoned due to difficulties passing the hysteroscope through the endocervical canal. The hysteroscopy was repeated in January 2004 and few intrauterine adhesions were reported. A Mirena IUS was inserted under the same general anaesthetic.
A month later she was admitted to the hospital with right upper quadrant pain and a problematic bleeding pattern. Ultrasound at this stage showed a normal size uterus but the Mirena IUS was not obviously in situ. It was assumed the Mirena IUS had fallen out and the patient was booked for vaginal hysterectomy, which was performed in September 2005.
In January 2007 was admitted to the hospital with right upper quadrant pain again and all investigations including chest X-ray, abdominal ultrasound scan and blood tests were normal. She had an upper GI endoscopy which showed a gastric ulcer (Cardia).
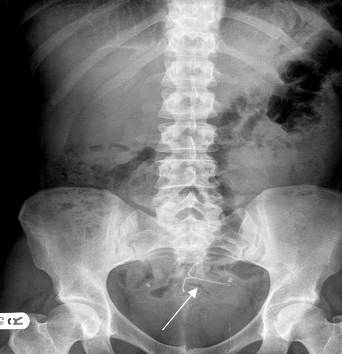
Fig.1 Abdominal X-Ray with the Mirena IUS (arrowed).
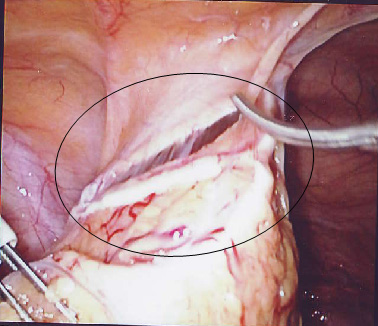
She was admitted again 1 year later with pelvic pain. An abdominal X-ray showed the lost IUS (fig. 1) and the CT scan showed the IUS to lie anteriorly under the rectus muscles and adjacent to the dome of the bladder. In April 2008 the IUS was retrieved laparoscopically. The omentum was adherent to anterior abdominal wall and the Mirena IUS was found in the omentum, (Fig.2). This was felt to be unlikely to be the cause of the pain. The IUS was removed easily from the abdominal cavity laparoscopically. The right tube and ovary were adherent to right pelvic side and they were freed up. The procedure was uneventful and the patient was discharged the same day and symptom free since.
Discussion
Uterine perforation is a serious, albeit rare, potential complication of Intrauterine contraceptive use. For informed consent, women should be informed that uterine perforation occurs in fewer than 1 in 1000 intrauterine LNG-IUS insertions4![]()
![]() Fig. 2 Mirena IUS (circled) within the omentum.
Fig. 2 Mirena IUS (circled) within the omentum.
The rate of perforation reported with the LNG-IUS in a large observational cohort study was 0.9 per 1000 insertions.5 Current guidelines recommend that advice regarding the management of problems arising with the LNG-IUS use4 is similar to that for IUD use3. The problems are suspected perforation, lost threads, abnormal bleeding, pregnancy, presence of actinomyces-like organisms, pelvic infection, and postmenopausal removal.
The Royal College of Obstetricians & Gynaecologists recommends6 that women who present with persistent menorrhagia, despite LNG-IUS use, should be advised to return for further assessment of the uterine cavity (biopsy or ultrasound scan) to exclude pathology.
If menorrhagia persists despite medical treatments, women should be re-examined.6 An assessment of the uterine cavity should be performed using ultrasound scan. An endometrial biopsy should be considered in all women with persistent menorrhagia. When indicated, a hysteroscopy allows the assessment of the uterine cavity and biopsy under local anaesthesia.6 The WHO Selected Practice Recommendations for Contraceptive Use (WHOSPR) 7does not specifically refer to the Mirena IUS. Follow-up 36 weeks following IUD insertion is recommended and the Clinical Effectiveness Unit (CEU) advises similar follow-up for women using the Mirena-IUS.
In this case report, the detection of the Mirena IUS inside the peritoneal cavity was noted nearly 4 years after the insertion and the perforation of the uterus. The patient had several admissions to the hospital under the care of gynaecologists or gastroentero-logists always complaining for upper or lower abdominal pain. She even had a vaginal hysterectomy. Had she undergone an abdominal hysterectomy, the Mirena IUS may have been noted at that time.
This case report clearly demonstrates that following an ultrasound report showing an empty uterus in a symptomatic patient, an abdominal X-ray should be performed to identify whether or not the IUS is inside the peritoneal cavity. Also, we need to be aware of the peritoneal adhesion potential of Mirena IUS it is expected to be low. In another case report8, an intraperitoneal Mirena IUS resulted in plasma levonorgestrel levels 10 times higher (4.7 nmol/l) than the plasma level of levonorgestrel observed with Mirena IUS placed in utero. This high plasma LNG level suppresses ovulation. Therefore, aside from the adhesion potential, a misplaced Mirena IUS should be removed when pregnancy is desired9, 10.
The authors conclude that judicious use of the abdominal X-ray can lead to the early detection of a migrated IUS and expedite early removal.
A thorough literature search of the Medline, Embase and Cochrane databases did not reveal case reports similar to this and also did not report any formal guidance as to the use of the Mirena IUS device following endometrial microwave ablation, but we did find an article regarding insertion of Mirena IUS, after endometrial resection.11
Endometrial resection is a surgical method to manage menorrhagia. Intrauterine scarring may occur following treatment, but it is not known if the risk of uterine perforation is increased.
The Clinical Effectiveness Unit (CEU) responded12 recently that the British National Formulary (BNF)13 suggested that intrauterine devices (IUDs) should be used with caution in severely scarred uteri.
The United Kingdom Medical Eligibility Criteria for Contraceptive Use (UKMEC)14 recommends that if women have a distorted uterine cavity (any congenital or acquired uterine abnormality distorting the uterine cavity in a manner that is incompatible with IUD insertion), then the IUD or the levonorgestrel-releasing intrauterine system (LNG-IUS) should not be used (UKMEC 4).
A narrative review paper on treatment after hysteroscopic surgery suggests that an acceptable post-operative method of contraception after endometrial ablation is the LNG-IUS, as it protects the endometrium and there is a high amenorrhoea rate.15 However, following successful endometrial ablation the uterine cavity is usually severely narrowed making insertion of IUS (or IUD) impossible and it would not normally be considered as an appropriate method in these circumstances. Significant bleeding would suggest failure of the procedure, and if IUS or IUD was to be considered it should only be done with hysteroscopic assistance by an experienced gynaecologist.
COMPETING INTERESTS
None Declared
AUTHOR DETAILS
SHAMBHU S MBChB Senior House Officer, Department of Obstetrics and Gynaecology, Hull Royal Infirmary, UK
PAPPAS M MBChB Specialist Registrar, Department of Obstetrics and Gynaecology, Hull Royal Infirmary, UK
CORRESPONDENCE: DR SHAMBHU S, Senior House Officer in Obstetrics and Gynaecology, Hull Royal Infirmary, Hull, UK
Email: siddesh@doctors.org.uk
References
-
Dawe F, Meltzer H. Contraception and Sexual Health, 2002. London, UK: Office for National Statistics, 2003; 149 http://www.statistics.gov.uk.
-
Schering Health Care Ltd. Mirena. 0053/0265, 18, 2002. http://www.schering.co.uk.
-
Faculty of Family Planning and Reproductive Health Care (FFPRHC). FFPRHC Guidance (January 2004). The copper intrauterine device as long-term contraception. J Fam Plann Reprod Health Care 2004; 30(1): 2942.
-
Faculty of Family Planning and Reproductive Health Care (FFPRHC). FFPRHC Guidance (January 2004). The levonorgestrel-releasing intrauterine system (LNG-IUS) in contraception and reproductive health. Journal of Family Planning and Reproductive Health Care 2004; 30(2): 99109
-
Zhou L, Harrison-Woolrych M, Coulter DM. Use of the New Zealand Intensive Medicines Monitoring Programme to study the levonorgestrel-releasing intrauterine device (Mirena). Pharmacoepidemiol Drug Saf 2003; 12: 371377
-
Royal College of Obstetricians and Gynaecologists (RCOG). The Management of Menorrhagia in Secondary Care. National Evidence- Based Clinical Guidelines. London, UK: RCOG, 1999.
-
World Health Organization (WHO). Selected Practice Recommendations for Contraceptive Use. Geneva, Switzerland: WHO, 2002.
-
Management of a perforated levonorgestrel - medicated intrauterine device: pharmacokinetic study: Case report Ronit Haimov-Kochman et al, Human Reproduction Vol.18, No.6 pp. 12311233, 2003.
-
Adoni, A. and Ben Chetrit, A. (1991) The management of intrauterine devices following uterine perforation. Contraception, 43, 7781.
-
Andersson, K., Ryde-Blomqvist, E., Lindell, K., Odlind, V. and Milsom, I. (1998) Perforations with intrauterine devices. Report from a Swedish survey. Contraception, 57, 251255.
-
Insertion of Mirena after Endometrial Resection in Patients with Adenomyosis . Hugo Maia, Jr. M.D, Amlia Maltez M.D.a, Genevieve Coelho M.D.a, Clia Athayde M.S.a and Elsimar M. Coutinho M.D. The Journal of the American Association of Gynecologic Laparoscopists. Volume 10, Issue 4, November 2003, Pages 512-516
-
MEMBERS ENQUIRY RESPONSE Enquiry Reference: 2268, Sent: 2nd April 2008 . Faculty of Family Planning and Reproductive Health Care. Clinical Effectiveness Unit
-
British National Formulary. BNF 54. 2007.
-
Faculty of Family Planning and Reproductive Health Care Clinical Effectiveness Unit. UK Medical Eligibility Criteria for Contraceptive Use. http://www.ffprhc.org.uk/admin/uploads/UKMEC200506.pdf. 2006.
-
Romer, T., Schmidt, T., and Foth, D. Pre- and postoperative hormonal treatment in patients with hysteroscopic surgery. Contributions to Gynecology and Obstetrics 20, 1-12. 2000.

The above article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.




