Liver Veno-Occlusive Disease (VOD) in a patient given 6-Thioguanine for Crohn’s Disease
Agata Salerno, Marco Vacante, Donatella Pollina, Benedetta Stancanelli, Silvia Martini, Ezio David, Lorenzo Malatino
Cite this article as: BJMP 2014;7(2):a711
|
|
Abstract 6-Thioguanine (6-TG) is being given to patients with Crohn's disease failing conventional immunosuppression, but cases of hepatotoxicity have been reported. We report the case of a patient who developed acute sinusoidal obstruction syndrome after 3 months of successful 6-TG treatment. A complete regression of liver injury was observed after withdrawal of 6-TG. Our case-report underscores the risk of hepatic injury due to the administration of 6-TG for Crohn’s disease. We strongly recommend alternative therapeutic options in patients intolerant or resistant to conventional thiopurines. Keywords: 6-thioguanine, Crohn’s disease, hepatotoxicity, veno-occlusive disease. |
A 44-yr-old patient with history of ileal Crohn’s disease was admitted to our Department because of asthenia, subclinical jaundice, painful hepatomegaly, fluid retention and ascites. In 2008 the patient was diagnosed with bladder cancer and was treated by surgical resection of the cancer and intravesical chemotherapy with mitomicyn C. In 2010 he was given azathioprine (AZA) at 2 mg/kg for Crohn’s disease and 3 months later he developed an increase in serum alkaline phosphatase, gamma-glutamyl transpeptidase and transaminases. He was then started on 1.5 mg/kg 6-mercaptopurine (6-MP) once daily. After 9 months he stopped 6-MP because of nausea, vomiting and abnormal liver function tests; 6-MP was therefore discontinued until the normalisation of markers of liver function. Two months later, when the transaminases were within the normal range, he received 6-thioguanine (6-TG) 25 mg a day, that was progressively increased to 80 mg a day. Three months later, the patient was referred to our Department with painful hepatomegaly, ascites and asthenia. Laboratory tests on admission revealed an elevation in AST 198 U/l and ALT 209 U/l. Total bilirubin was 3 mg/dl (direct bilirubin 1.5 mg/dl), LDH 784 U/l, alkaline phosphatase 191 U/l and ammonia 112 umol/l. Virological markers (HBsAg, HBcAb, anti HCV, HBV DNA) were negative. Patient was apyrexial, showed normal blood pressure (130/80 mmHg), tachycardia (110 bpm) and 97% SaO2 on room air. Physical examination revealed right hypochondrial tenderness, abdominal distension and shifting dullness, suggesting the presence of ascites. The rest of the physical examination was unremarkable. An echo-Doppler evaluation revealed thin linear suprahepatic veins and confirmed the presence of ascites. A CT scan of the abdomen showed hepatomegaly with dishomogeneous enhancement after dye injection (mosaic pattern). There was no evidence of any venous thrombosis or splenomegaly (Figure 1A); 6-TG was withdrawn empirically and the patient was started on therapy with albumin 25 g/day and spironolactone 200 mg/day. The average serum Na+ level during diuretic treatment was 134 mEq/l. An abdominal paracentesis of two litres was necessary, due to the progressive increase of ascites.
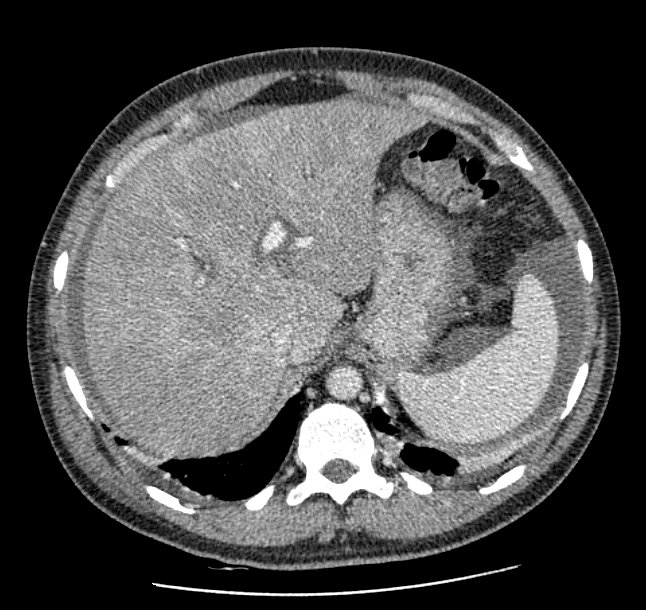
FIGURE 1A. CT scan of the abdomen on admission: Dishomogeneous enhancement of the liver after dye injection (mosaic pattern) (arrow). Suprahepatic veins are not detectable.
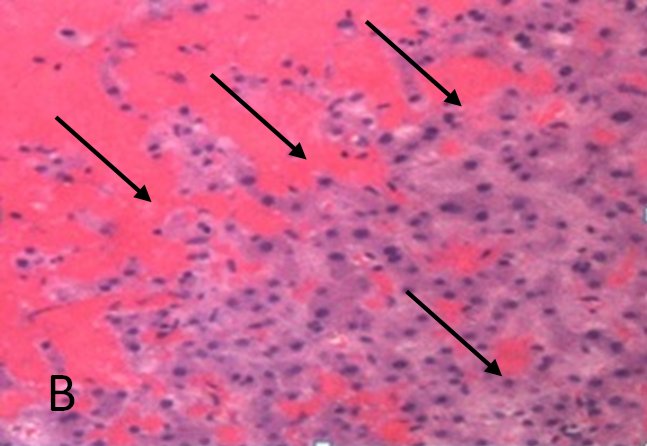
FIGURE 1B. Histological pattern of the liver biopsy specimen: marked centrilobular congestion (arrows) with hepatocyte dropout. There is no evidence of centrolobular veins thrombosis.
A routine laboratory investigation of ascitic fluid showed < 500 leukocytes/µL and < 250 polymorphonuclear leukocytes (PMNs)/µL. The ascitic fluid total protein level was 2.1 g/dl and serum-ascites albumin gradient (SAAG) was > 1.1 g/dL. No neoplastic cells were found. A transjugular liver biopsy was then performed, showing marked centrilobular hemorrhage with hepatocyte necrosis. There was mild ductular reaction, with no evidence of centrilobular vein thrombosis. The histologic diagnosis confirmed veno-occlusive disease (VOD) (Figure 1B). Screening for thrombophilia was also done, showing low levels of serum protein C and protein S. There was no mutation of JAK-2 V617F. The patient was then treated with a hyposodic diet, mild hydric restriction, enoxaparin,spironolactone, lactulose and omeprazole. He was discharged two weeks later, and after 3 months a complete regression of ascites and hepatomegaly occurred, and echography of the liver was unremarkable (Figure 2A and 2B).
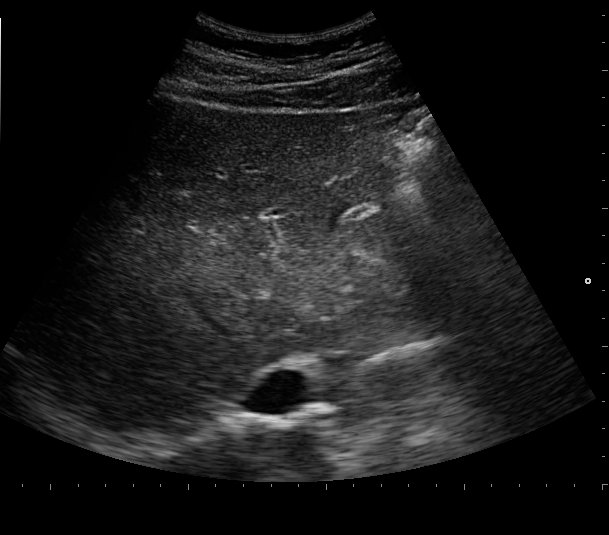
FIGURE 2A. Echography of the liver at follow up. No evidence of ascites.
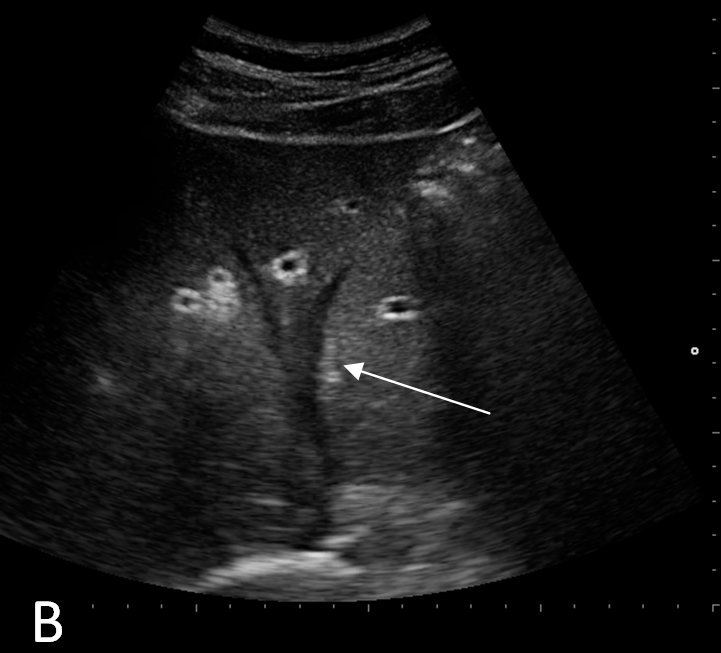
FIGURE 2B. Echography of the liver at follow up. No evidence of ascites. Suprahepatic veins are detectable (arrow)
Discussion
Although VOD was known among complications of 6-TG in childhood, this case-report emphasises the occurrence of VOD in adults with Crohn’s disease, as first described by Kane et al. in 20041. The thiopurine drugs were developed more than 50 years ago, and 6-MP was first used as a drug in 19522. Since then, 6-MP and 6-TG have been widely used to treat acute lymphoblastic leukemia in children. VOD mimicking Budd-Chiari like disease was then described as a frequent complication of 6-TG in pediatric patients given the drug for lymphoblastic leukaemia. Later on, in 1976, Griner et al. described the cases of two adult male patients with acute leukaemia developing a fatal Budd-Chiari-like disease while receiving 6-TG3. Since patients were given 6-TG plus cytosine arabinoside, authors were unable to ascribe this complication solely to 6-TG3. VOD exclusively related to 6-TG was first described by Gill et al., who observed a clinically reversible liver VOD developing in a young man with acute lymphocytic leukemia after 10 month administration of 6-TG4. Furthermore, sinusoidal obstruction was also reported in a patient with psoriasis treated with 6-TG and other cytotoxic therapy5. In 2006, a European 6-TG Working Party established that 6-TG should be considered a rescue drug in stringently defined indications in inflammatory bowel diseases (IBD). The indication for administration of 6-TG should only include its use for maintenance therapy as well as intolerance and/or resistance to aminosalicylates, azathioprine, 6-mercaptopurine, methotrexate and infliximab. Moreover, 6-TG must be withdrawn in case of overt or histologically proven hepatotoxicity6. Although Ansari et al 7 found no nodular regenerative hyperplasia (NRH) in the liver of patients given 6-TG, Dubinsky et al.8 described NHR as a common finding in 6-TG-treated patients with inflammatory bowel disease in the absence of VOD. By contrast, in our case report we showed histological pattern of VOD and, in accord with Gisbert et al.9, would suggest that 6-TG should not be administered out of a clinical trial setting. Given that the proportion of patients with Crohn’s disease achieving an improvement of symptoms during 6-TG treatment is similar to that after methotrexate10 or infliximab6, these drugs should therefore be considered as second line therapy in patients intolerant or resistant to azathioprine and 6-mercaptopurine.
|
Competing Interests None declared Author Details AGATA SALERNO MD, Department of Internal Medicine, University of Catania, c/o Cannizzaro Hospital, Via Messina 829, 95126 Catania, Italy. MARCO VACANTE, MD, Department of Internal Medicine, University of Catania, c/o Cannizzaro Hospital, Via Messina 829, 95126 Catania, Italy. DONATELLA POLLINA, MD, Department of Internal Medicine, University of Catania, c/o Cannizzaro Hospital, Via Messina 829, 95126 Catania, Italy. BENEDETTA STANCANELLI, MD, Department of Internal Medicine, University of Catania, c/o Cannizzaro Hospital, Via Messina 829, 95126 Catania, Italy. SILVIA MARTINI, MD,SSCVD Insufficienza Epatica e Trapianto, Azienda Ospedaliera Città della Salute e della Scienza – Molinette, C.so Bramante 88, 10126 Turin, Italy. EZIO DAVID, MD,SCDU II Anatomia Patologica, Azienda Ospedaliera Città della Salute e della Scienza – Molinette, C.so Bramante 88, 10126 Turin, Italy. LORENZO MALATINO, MD, PROFESSOR OF MEDICINE Department of Internal Medicine, University of Catania, c/o Cannizzaro Hospital, Via Messina 829, 95126 Catania, Italy. CORRESPONDENCE: MARCO VACANTE, Department of Internal Medicine, University of Catania, c/o Cannizzaro Hospital, Via Messina 829, 95126 Catania, Italy. Email: marcovacante@yahoo.it |
References
- Kane S, Cohen SM, Hart J. Acute sinusoidal obstruction syndrome after 6-thioguanine therapy for Crohn's disease. Inflamm Bowel Dis. 2004;10:652-4.
- Elion G, Burgi E, Hitchings G. Studies on condensed pyrimidine systems, IX. The synthesis of some 6-substituted purines. J Am Chem Soc 1952;74:411-4.
- Griner PF, Elbadawi A, Packman CH. Veno-occlusive disease of the liver after chemotherapy of acute leukemia. Report of two cases.Ann Intern Med. 1976;85:578-82.
- Gill RA, Onstad GR, Cardamone JM, Maneval DC, Sumner HW. Hepatic veno-occlusive disease caused by 6-thioguanine.Ann Intern Med. 1982;96:58-60.
- Kao NL, Rosenblate HJ. 6-Thioguanine therapy for psoriasis causing toxic hepatic venoocclusive disease. J Am Acad Dermatol. 1993;28:1017-1018.
- de Boer NK, Reinisch W, Teml A, van Bodegraven AA, Schwab M, Lukas M, et al; Dutch 6-TG working group. 6-Thioguanine treatment in inflammatory bowel disease: a critical appraisal by a European 6-TG working party.Digestion. 2006;73:25-31.
- Ansari A, Elliott T, Fong F, Arenas-Hernandez M, Rottenberg G, Portmann B, et al. Further experience with the use of 6-thioguanine in patients with Crohn's disease.Inflamm Bowel Dis. 2008;14:1399-405.
- Dubinsky MC, Vasiliauskas EA, Singh H, Abreu MT, Papadakis KA, Tran T, et al. 6-thioguanine can cause serious liver injury in inflammatory bowel disease patients.Gastroenterology. 2003;125:298-303.
- Gisbert JP, González-Lama Y, Maté J. Thiopurine-induced liver injury in patients with inflammatory bowel disease: a systematic review. Am J Gastroenterol. 2007;102:1518-27.
- Feagan BG, Rochon J, Fedorak RN, Irvine EJ, Wild G, Sutherland L, et al. Methotrexate for the treatment of Crohn's disease. The North American Crohn's Study Group Investigators. N Engl J Med. 1995;332:292-7.

The above article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.




