Latest diagnosis and management of diverticulitis
Stephen O’Neill, Phillip Ross, Philip McGarry and Satheesh Yalamarthi
Cite this article as: BJMP 2011;4(4):a443
|
|
Abstract Diverticular disease is extremely common especially amongst the elderly. It mainly presents as sigmoid diverticulitis but there is potential for serious complications. In the acute setting Computed Tomography is the gold standard investigation and helps classify the stage. Evidence to support outpatient treatment of uncomplicated diverticulitis is appearing however hospital admission and treatment with intravenous antibiotics is often required and is highly effective. The decision to proceed with elective surgery is judged on an individual basis with a long-term conservative approach suitable for most. For elective surgery there is evidence to advocate a laparoscopic approach. In Hinchey stage III or IV disease, laparotomy followed by either a Hartmann’s procedure or ideally, a resection followed by primary anastomosis may be required. Radiologically guided drainage of an abscess is an established alternative and laparoscopic lavage is another less invasive option that has emerged. Following successful acute medical management, colonoscopy is usually performed several weeks after resolution to rule out other colonic pathology. Keywords: Diverticulitis, Diagnosis, Management, Surgery |
Introduction
A colonic diverticulum is defined as a sac-like protrusion of mucosa through the muscular component of the colonic wall1. The terms “diverticulosis” and “diverticular disease” are used to express the presence of diverticula without associated inflammation. While the term “diverticulitis” indicates there is inflammation of a diverticulum or diverticula, which is commonly accompanied by either microscopic or macroscopic perforation2.
In the developed world, diverticular disease of the colon is widespread and in those aged over 65 years of age it is present in greater than 65%3. The incidence increases dramatically with time and while only 5% of the western population are affected in the fifth decade this rises steeply to over 50% by the eight decade and 60% in the ninth 4.
Although diverticulosis is extremely common, complications requiring surgery only occur in 1% of patients overall 5 and 10% of those admitted to hospital as an emergency for treatment6. Despite this, there is a substantial healthcare burden inflicted by diverticular disease and within the United States alone it accounts for 312,000 hospital admissions, 1.5 million days of inpatient treatment and a total estimated cost of 2.6 billion dollars per annum 7.
The aetiology of the diverticulosis is poorly understood but it is probably a multi-factorial process involving dietary habits (specifically low fibre intake) as well as changes in colonic pressure, motility and wall structure that are associated with ageing8. The pathogenesis of diverticulitis is also uncertain, however stasis or obstruction in a narrow necked diverticulum leading to overgrowth of pathogens and local tissue ischemia is thought likely 2.
This review will discuss the common presentations, investigations and current treatment strategies utilised in the management of acute diverticulitis and its complications as well as providing an up to date synopsis of existing recommendations for follow up and prevention.
Symptoms and Signs
In Western nations, diverticula are most commonly situated in the left colon9 and 99% of patients will have some element of sigmoid involvement10. Therefore patients commonly present with sigmoid diverticulitis that typically displays features of left iliac fossa pain and fever with raised inflammatory markers (see below). Physical exam will disclose left lower quadrant peritonism for simple disease, but in complicated cases physical examination findings may reveal a palpable abdominal mass, evidence of fistulas or obstruction, or widespread peritonitis11.
In cases of complicated diverticulosis, a stricture may lead to obstructive symptoms with complaints of nausea, vomiting and distension being present. If a fistula has developed, a history of recurrent urinary tract infection, pneumaturia and faecaluria may also be elicited12. In a female with a previous history of hysterectomy suspicion will be further raised as colovesical and colovaginal fistulas are rare in females with their uterus in place. If a patient reports passing stools per vagina, insertion of a vaginal speculum and inspection may confirm this latter diagnosis12.
Differential diagnosis
The differential diagnosis for diverticulitis and its complications is extensive and includes irritable bowel syndrome, inflammatory bowel disease, ischaemic or infective colitis, pelvic inflammatory disease and malignancy. It is obviously most imperative to exclude the latter differential 4, particularly in the case of a stricture that is impassable on colonoscopy, as many of these specimens following resection (32% in one series13) will transpire to be adenocarcinoma4. It should also be noted that sigmoid diverticulitis may also masquerade as acute appendicitis if the colon is long and redundant or otherwise situated within the abdomen or pelvis such that the inflamed segment lies in the suprapubic region, right iliac fossa or McBurney’s point2.
Complications
Although diverticulosis is present in nearly two thirds of the elderly population, the vast majority of patients will remain entirely asymptomatic. Even so, an estimated 20% of those affected will manifest symptomatology, mainly as diverticulitis, but potentially with further complications of perforation, abscesses, fistulas, and obstruction, as well as bleeding per rectum6.
The European Association for Endoscopic Surgeons (EAES) developed a classification scheme based upon the severity of diverticulitis, which broadly classifies patients into either simple symptomatic or complicated disease (Table 1)14. Where an abscess or perforation develops the Hinchey classification is used as a staging tool and can provide prognostic information on the likely outcome (Table 2)15.
Table 1 - European Association for Endoscopic Surgeons classification system for diverticulitis 14
| Grade of disease | Clinical explanation of grade | Clinical state of the patient |
| I | Symptomatic uncomplicated disease | Pyrexia, abdominal pain, CT findings consistent with diverticulitis |
| II | Recurrent symptomatic disease | Recurrence of Grade I |
| III | Complicated disease | Bleeding, abscess formation, phlegmon, colonic perforation, purulent and faecal peritonitis, stricturing, fistula and obstruction |
Table 2 – Hinchey classification of perforated diverticulitis 15
| Hinchey stage | Features of disease | Risk of death71 |
| Stage I* | Diverticulitis with a pericolic abscess | 5% |
| Stage II** | Diverticulitis with a distant abscess (this may be retroperitoneal or pelvic) | 5% |
| Stage III | Purulent peritonitis | 13% |
| Stage IV | Faecal peritonitis | 43% |
* Stage I has been divided into Ia Phlegmon and Ib confined pericolic abscess in later modifications38 72
** Stage II has been divided into IIa abscesses amenable to percutaneous drainage and IIb complex abscess with or without fistula in later modifications14 73
Perforation is probably the most feared complication and the annual prevalence of perforated diverticulitis within a northern European population is currently thought to stand at 3.8 per 100,000 of the population, which is a figure that is increasing16. Despite this only 1-2% of patients who attend for urgent assessment and treatment will have a gross perforation2 but for 80% this will be their first presentation so a high index of suspicion is still required17.
Blood investigations
In clinical practice, inflammatory markers, commonly the White Blood Cell (WBC) count and C-Reactive Protein (CRP) level, are frequently employed to assist in diagnosing diverticulitis and its complications. In a recent retrospective study, a White Blood Cell (WBC) count >10,000/μL was present in 62% of patients with Computed Tomography (CT) confirmed diverticulitis and the presence of leukocytosis was significantly more common in patients with diverticulitis and associated perforation than without (86% v 65%, p=0.01)18.
CRP has also been shown to be of considerable benefit in the diagnosis of acute left sided colonic diverticulitis 19. A recently established diagnostic nomogram with a reported accuracy of 86% that was developed to improve the clinical diagnosis of diverticulitis includes an elevated CRP >50mg/l as well other variables including age, previous episodes, aggravation of pain on movement, absence of vomiting and localization of symptoms and tenderness in the left iliac fossa19.
In addition, it has been demonstrated that in acute sigmoid diverticulitis a CRP below 50mg/l is unlikely to correlate with an associated perforation (negative predictive value 79%) while a CRP above 200mg/l is an indicator that the patient may have a perforation (positive predictive value 69%)20. In this latter study, CRP also had the highest diagnostic accuracy in diagnosing perforation in acute sigmoid diverticulitis across a range of parameters assessed that included WBC count as well as less commonly used tests like bilirubin and alkaline phosphatase20.
Imaging investigations
In the acute phase of diverticulitis the extent of the extramural component of inflammation is more important than the degree of the intramural inflammation and as such CT associated with the use of intravenous and oral contrast and, in ideal conditions, rectal contrast is the gold standard means of investigation21.
CT can accurately identify extra-luminal complications such as an abscess, phlegmon, adjacent organ involvement, or fistula, as well as recognising other alternative diagnoses such as appendicitis, pelvic inflammatory disease, tubo-ovarian abscess or inflammatory bowel disease22.
The two most frequent signs of diverticulitis on CT are bowel wall thickening (96%) and fat stranding (95%) (Figure 1) with less common but highly specific signs including fascial thickening (50%), free fluid (45%), and the presence of inflamed diverticula (43%) 23. Specifically, abscess formation (Figure 2a and b) and extracolonic air or contrast (Figure 3a and b) are findings that are known to predict severity as summarised in the CT classification system developed by Ambrosetti et al 24.
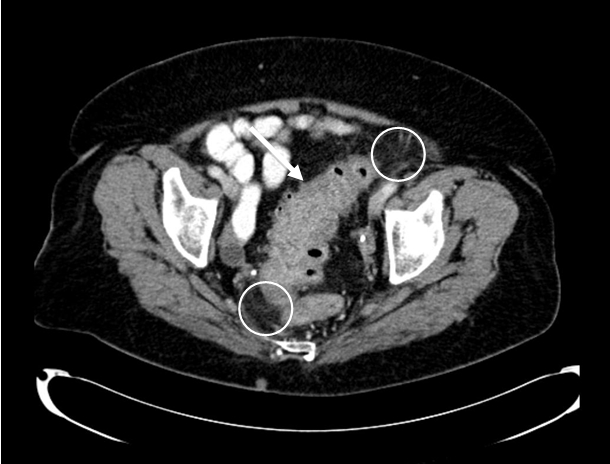
Figure 1 - Sigmoid diverticulitis: sigmoid colon with multiple diverticula, significant mural thickening (arrow) and pericolic fat stranding (circles)
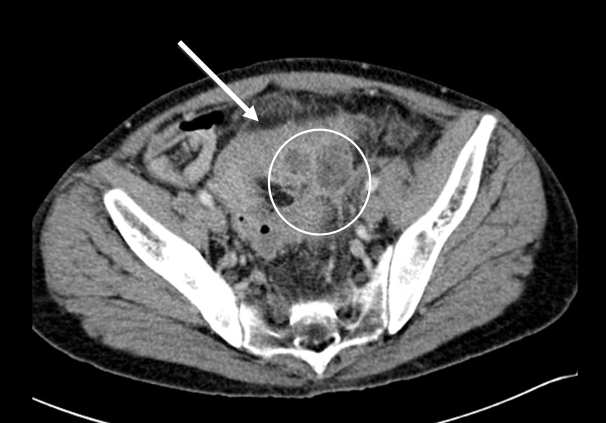
Figure 2a - Sigmoid diverticulitis with abscess formation: sigmoid colon displaying diverticulosis mural thickening, and pericolic fat stranding (arrow). Adjacent low attenuation, septated collection (circle) representing abscess formation.
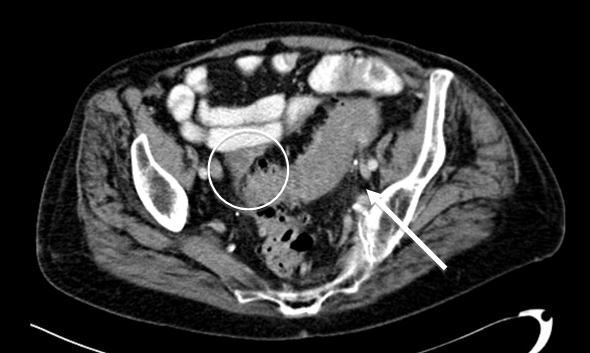
Figure 2b - Sigmoid diverticulitis with abscess formation: sigmoid colon displaying mural thickening, diverticulosis and pericolic fat stranding (arrow). Adjacent low attenuation, septated collection (circle) representing abscess formation, with adhesion noted to adjacent small bowel loops.
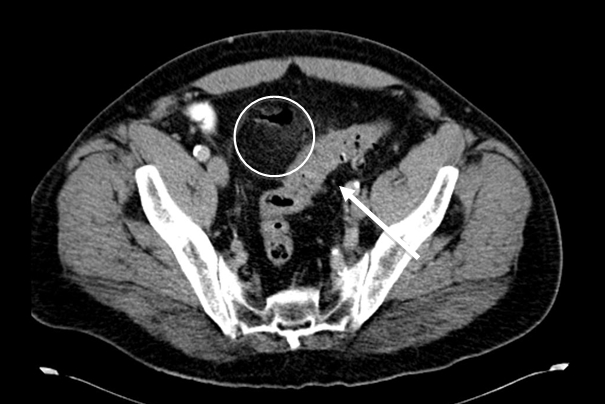
Figure 3a - Perforated sigmoid diverticulitis: sigmoid colon displaying diverticulosis and mural thickening (arrow) with adjacent collection of intra-abdominal free air and adjacent inflammatory fat stranding (circle), representing active diverticulitis with perforation.
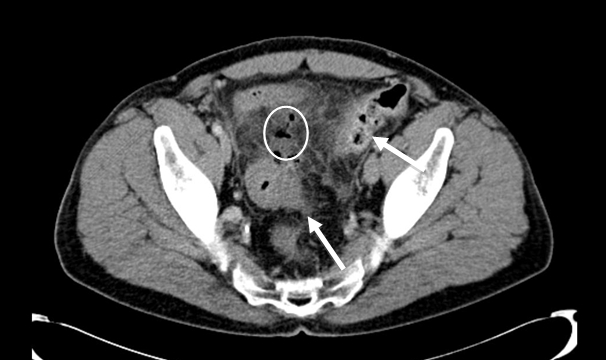
Figure 3b - Perforated sigmoid diverticulitis: sigmoid colon displaying diverticulosis, mural thickening and pericolic inflammatory fat stranding (arrow) with adjacent collection of intra-abdominal free air and adjacent inflammatory fat stranding (circle), again representative of active diverticulitis with perforation.
However despite CT having a reported sensitivity of 97%, specificity of 98%, and global accuracy of 98%25, a misdiagnosis of diverticulitis in cancer patients is relatively common and occurs in 5% of cases21. Therefore investigation of the colonic lumen by endoscopic means or barium enema after the acute attack is mandatory4 but avoided in the initial stages for fear of perforation and exacerbation of the disease2.
In expert hands ultrasound is the next best alternative investigation with a reported sensitivity of 94%26. It has been supported by a recent systematic review27 as well as current practice guidance4 and in critically ill patients it avoids the use of intravenous and intra-luminal contrast21. However it is rarely used in practice as it is operator dependent21 and for it to be accurately utilised it requires a highly skilled/trained individual to be available at all times28.
The other practical alternative to CT is a hydro-soluble contrast enema, however this investigation is significantly inferior both in terms of sensitivity (98 v 92%, p<0.01) and evaluation of the severity of inflammation (26 v 9%, p<0.02)29. While Magnetic resonance imaging (MRI) has a good sensitivity of 94% and a specificity of 87%30, in the acute setting it may be impractical both in terms of examination time and patient co-operation21. Finally, laparoscopy can also be helpful for diagnostic purposes but again in practical terms, with the increasing availability of cross-sectional imaging, it is rarely required for this purpose4.
Outpatient treatment
Evidence for successful and economical outpatient treatment of uncomplicated diverticulitis is beginning to emerge. In a prospective study of 70 patients classified on the basis of an ultrasound examination as having mild-to-moderate acute colonic diverticulitis (as defined by either limited inflammation within a diverticulum extending up to an abscess < 2 cm in diameter), 68 patients were successfully treated with oral antibiotics with an initial liquid diet and this led to a cost saving on inpatient treatment of 80%31.
In a further retrospective analysis, among a cohort of patients who were referred for outpatient treatment it was found that such treatment was effective for 94% of patients, with women and those with free fluid on CT scan appearing to be at higher risk for treatment failure32.
In reality the prospect of outpatient treatment in uncomplicated cases of acute diverticulitis is determined largely by access to the necessary investigative tools for accurate diagnosis and staging of disease, the general fitness of the patient, their ability to maintain adequate oral intake, the possibility of further outpatient review, patient compliance with medications, satisfactory social support and ability to plan for endoscopic follow up21.
In broad terms, if symptoms are not severe and the patient has no significant co-morbidities and is compliant with medical treatment, then a course of broad spectrum antibiotics can be administered orally on an outpatient basis and the patient followed up at subsequent outpatient clinics. However if the patient is systemically unwell, elderly, has significant co-morbidities or there are any other concerns it is safer to arrange for a hospital admission and treatment with intravenous antibiotics12.
Conservative inpatient treatment
Simple diverticulitis requiring hospital admission is usually treated by rehydration, symptomatic relief and intravenous antibiotics. Most patients with uncomplicated disease respond well to medical treatment and generally experience significant improvement in their abdominal pain, temperature and inflammatory markers within two days of initiation of antibiotic treatment33. If this is not the case or there is clinical concern a repeat CT is advocated and operative intervention or percutaneous drainage considered (see below)2.
It should be noted at this stage while the use of broad spectrum antibiotics in acute uncomplicated diverticulitis is supported by guidelines34 there is no actual evidence mandating the routine use of antibiotics in mild uncomplicated diverticulitis35 and in some European countries it is not routine36.
High-quality evidence regarding the most effective type of antibiotic is also lacking35. However anaerobic bacteria (usually bacteroides, clostridium, fusobacterium and peptostreptococcus) are the most commonly cultured organisms with gram-negative aerobes, especially Escherichia coli, and facultative gram-positives, such as streptococci, often grown as well37. Therefore coverage against both Gram-negative and anaerobic bacteria is widely advocated2 21 38.
If combination antibiotics are selected, Metronidazole provides excellent anaerobic cover with less risk of clostridium difficle infection than alternatives4. However use of single agent may be more cost effective39. Local protocols are likely to influence selection but the patient may be safely switched from intravenous to oral therapy when they can tolerate a diet and oral medicines22 as intravenous antibiotics are not felt to be vastly superior40. Seven to ten days of antibiotic therapy is an acceptable treatment period22 however evidence is emerging to support shorter courses41.
Elective surgery
In a recent position statement from the Association of Coloproctology of Great Britain and Ireland (ASCPGBI) it was concluded that the majority of patients, whether young or old, presenting with acute diverticulitis could be managed with a conservative, medical approach in the longer term. Previous blanket recommendations for elective resection e.g. following two acute episodes of diverticulitis14 were challenged in this statement and it was proposed that the decision on elective resection should be made on an individual basis4. The traditional practice of waiting for a period of 4-6 weeks after a diverticulitis attack before performing an elective operation was not disputed12.
Surgery in the elective setting can be by either an open or laparoscopic technique with a recent randomised trial identifying a 27% reduction in major morbidity42 along with less pain, improved quality of life and shorter hospitalization at the cost of a longer operating time with the laparoscopic approach43. In expert centres conversion rates as low as 2.8% and median hospitals stays of 4 days can be achieved44 and individual case reports of resections using single laparoscopic port access have also emerged45. However if a laparoscopic resection is considered, it is currently recommended that patients should be treated after full recovery from the acute episode of inflammation as there is evidence to suggest lower complication and conversion rates can be achieved4.
The principles for both approaches are the same. A colorectal anastomosis is a predictor of lower recurrence rates after elective sigmoid resection for uncomplicated diverticulitis46. Therefore it is recommended that the distal resection margin is taken onto the rectum as opposed to the distal sigmoid and the splenic flexure is fully mobilised to facilitate this4, however in the case of a long redundant left colon this may not be necessary12. The proximal resection margin is less clear but should be made onto soft compliant bowel4 34. Often it is possible to identify the ureters intra-operatively however, there may be cases of complicated diverticulitis in which the extent and degree of inflammatory changes warrant the use of pre-operatively placed ureteric stents to help aid their identification and avoid injury12.
Emergency surgery for complicated diverticulitis
The indications for emergency operative intervention in acute diverticulitis include the presence of generalised peritonitis, uncontained visceral perforation, gross uncontrollable sepsis, a large undrainable or inaccessible abscess, bowel obstruction and lack of improvement or clinical deterioration with initial medical management 2.
Historically, perforated diverticulitis was treated with a three-stage procedure consisting of faecal diversion with a stoma, resection of the diseased segment of bowel, followed by takedown of the stoma and restoration of intestinal continuity. This then shifted to performing a Hartmann’s procedure which includes a primary resection of the diseased segment and end colostomy followed by subsequent colostomy reversal at a second operation11. In this case reconstruction generally involves a second laparotomy because although laparoscopic reconstruction is effective, it is infrequently performed47-48. As a result reversal is often permanently deferred.
In selected cases the ideal therapeutic option in colonic perforation is a one-stage procedure with resection followed by primary anastomosis, which adds the benefits of being a definitive treatment with the avoidance of the morbidity and mortality associated with a stoma and its reversal49. A protective ileostomy after resection and primary anastomosis is viewed as a valid additional step in patients at high risk of an anastomotic leak (immunosuppression, American Society of Anaesthesiologists (ASA) grade IV, faecal peritonitis)21 but a Hartmann’s procedure may also be selected.
Particularly in cases where there is a stricture causing obstruction and significant faecal loading, a resection in conjunction with on-table colonic lavage and primary anastomosis may be used. This technique has also been described as facilitating a primary anastomosis in the case of a perforation50. However in certain patients with obstruction depending on the viability of the proximal colon a subtotal colectomy with ileorectal anastomosis may be required12 and because small-bowel obstruction may also occur, especially in the presence of a large diverticular abscess, this may also warrant further treatment2.
The use of endoscopic colonic stenting as a treatment of acute obstruction of the large bowel secondary to colonic cancer has been well documented in the literature either as a definitive procedure or as a bridge to surgery and can effectively decompresses the obstructed colon in 90% of cases51. However the use of stents in benign disease is less well documented , with it used mainly as a bridge to surgery52 and because is associated with a higher incidence of complications in acute diverticular disease53 it cannot as yet be recommended.
Laparoscopic surgery in the emergency setting
There have been a number of recent reports of laparoscopic lavage with or without the placement of an intra-abdominal drain for patients with acute diverticulitis and perforation, with the reported advantages including the avoidance of an acute resection and the possibility of a stoma 4. The evidence that has been produced thus far to support its case is highly promising.
A recent systematic review of laparoscopic lavage for perforated colonic diverticulitis identified two prospective cohort studies, nine retrospective case series and two case reports with 231patients and the vast majority of patients (77%) had Hinchey grade III purulent peritonitis. Laparoscopic peritoneal lavage successfully controlled abdominal and systemic sepsis in 95.7% of patients, mortality was 1.7%, morbidity 10.4% and only four (1.7%) patients received a colostomy54.
In the largest series in the literature to date, Myers et al reported 100 patients with perforated diverticulitis and generalised peritonitis. Eight patients with Hinchey IV disease required conversion to an open procedure, with the overall mortality being 4% and recurrence rates only 2% over a median time period of 36 months55.
Percutaneous therapy
The appropriate management of diverticular abscesses is a matter of some debate. However according to the American Society of Colon and Rectal Surgeons (ASCRS) radiologically guided percutaneous drainage is usually the most appropriate treatment for patients with a large diverticular abscess as it avoids the need for emergency surgery and possibility of a colostomy34.
When the abscess diameter is over 5 cm, percutaneous CT guided drainage, in combination with antibiotics, is the standard treatment and offers rapid improvement in symptoms in over 90% of cases, albeit with a high recurrence rate in more severe cases38 and higher likelihood of surgery being needed in those involving the pelvis56.
In practical terms diverticular abscesses less than 3 cm in diameter usually cannot be successfully drained, as the diameter of the pigtail of most drainage catheters will be a similar dimension28. Also for smaller abscesses21, especially those less than 2cm resolution usually occurs with the use intravenous antibiotics alone34. However if a drain is sited it is advisable that before it is removed, resolution of the abscess should be confirmed and a potential bowel fistula excluded by a further contrast study28.
Finally, diverticular disease of the colon is also a relatively common cause of acute lower gastrointestinal bleeding and is in fact the diagnosis in 23% of cases57. This usually settles with conservative management but if the bleeding is profuse angiography and endovascular intervention may be helpful, with surgery very rarely required for this indication4.
Follow up
Following successful medical management of an acute episode of diverticulitis, colonoscopy, flexible sigmoidoscopy or barium enema should be performed several weeks after the resolution of symptoms to confirm the diagnosis and rule out other colonic pathology such as malignancy, inflammatory bowel disease, or ischemia22.
Following surgery there is reported to be a high incidence of the order of 25% for recurrent symptoms, which is put down to the diagnostic overlap that exists with irritable bowel syndrome58. However any suspicion of recurrent diverticulitis following surgical resection should be confirmed by CT scan after which antibiotic treatment should be initiated, as for a case of primary uncomplicated disease12. If this is excluded the high incidence (17.6%) of symptomatic anastomotic stenosis after elective laparoscopic sigmoidectomy should be borne in mind with the possibility of endoscopic dilatation considered if applicable59.
| Summary points |
|
Lifestyle modifications and prevention
Following treatment weight loss, rationalisation of certain medications and exercise are recommended as obesity is significantly associated with an increased incidence of both diverticular bleeding and diverticulitis60, as are non-steroidal anti-inflammatory drugs and paracetamol61, with physical activity significantly associated with a reduction in the risk of complications62.
Whilst dietary fibre, particularly cellulose63, is recommended22 the evidence that supports these recommendations is not particularly strong64. However foodstuffs such as nuts, seeds, popcorn and corn that are usually discouraged have no evidence to support the theory that they may lead to increased complications65.
Small studies without control groups suggest that probiotics may have a positive effect on the recurrence of symptomatic diverticular disease66-67. Long term administration of the non-absorbable antibiotic Rifaxamin has also been used with reported success68 as has the anti-inflammatory mesalazine69. However none of these medications have a strong evidence base and as a result are not in routine use70.
|
Competing Interests None declared Author Details S O’NEILL, Surgical Registrar MB BCh BAO (dist) MSc (dist) MRCSEd Department of Colorectal Surgery, Queen Margaret Hospital, Dunfermline. P ROSS, Medical Student, Queens University, Belfast. P MCGARRY, Radiology Registrar MB BCh BAO (dist), Department of Radiology, Altnagelvin Hospital, Londonderry. S YALAMARTHI, Consultant Surgeon MS FRCS, Department of Colorectal Surgery, Queen Margaret Hospital, Dunfermline CORRESPONDENCE: Mr STEPHEN O’NEILL, 6 Dean Park Mews, Edinburgh EH4 1EF. Email: stephenoneill@doctors.org.uk |
References
1. Young-Fadok TM, Roberts PL, Spencer MP et al. Colonic diverticular disease. Curr Probl Surg 2000;37(7):457-514.
2. Jacobs DO. Clinical practice. Diverticulitis. N Engl J Med 2007;357(20):2057-66.
3. West AB. The pathology of diverticulitis. J Clin Gastroenterol 2008;42(10):1137-8.
4. Fozard JB, Armitage NC, Schofield JBet al. ACPGBI position statement on elective resection for diverticulitis. Colorectal Dis 2011;13 Suppl 3:1-11.
5. Roberts PL, Veidenheimer MC. Current management of diverticulitis. Adv Surg 1994;27:189-208.
6. Stollman NH, Raskin JB. Diverticular disease of the colon. J Clin Gastroenterol 1999;29(3):241-52.
7. Etzioni DA, Mack TM, Beart RW, Jr.et al. Diverticulitis in the United States: 1998-2005: changing patterns of disease and treatment. Ann Surg 2009;249(2):210-7.
8. Heise CP. Epidemiology and pathogenesis of diverticular disease. J Gastrointest Surg 2008;12(8):1309-11.
9. Vermeulen J, van der Harst E, Lange JF. Pathophysiology and prevention of diverticulitis and perforation. Neth J Med 2010;68(10):303-9.
10. Hughes LE. Postmortem survey of diverticular disease of the colon. II. The muscular abnormality of the sigmoid colon. Gut 1969;10(5):344-51.
11. Lopez DE, Brown CV. Diverticulitis: the most common colon emergency for the acute care surgeon. Scand J Surg 2010;99(2):86-9.
12. Stocchi L. Current indications and role of surgery in the management of sigmoid diverticulitis. World J Gastroenterol 2010;16(7):804-17.
13. King DW, Lubowski DZ, Armstrong AS. Sigmoid stricture at colonoscopy--an indication for surgery. Int J Colorectal Dis 1990;5(3):161-3.
14. Kohler L, Sauerland S, Neugebauer E. Diagnosis and treatment of diverticular disease: results of a consensus development conference. The Scientific Committee of the European Association for Endoscopic Surgery. Surg Endosc 1999;13(4):430-6.
15. Hinchey EJ, Schaal PG, Richards GK. Treatment of perforated diverticular disease of the colon. Adv Surg 1978;12:85-109.
16. Makela J, Kiviniemi H, Laitinen S. Prevalence of perforated sigmoid diverticulitis is increasing. Dis Colon Rectum 2002;45(7):955-61.
17. Hart AR, Kennedy HJ, Stebbings WSet al. How frequently do large bowel diverticula perforate? An incidence and cross-sectional study. Eur J Gastroenterol Hepatol 2000;12(6):661-5.
18. Mounday A, Aubin C, Lewis L. Absence of fever and elevated white blood count does not exclude the diagnosis of diverticulitis as determined by CT in the emergency department. Ann Emerg Med 2007;50(3):S77.
19. Andeweg CS, Knobben L, Hendriks JCet al. How to diagnose acute left-sided colonic diverticulitis: proposal for a clinical scoring system. Ann Surg 2011;253(5):940-6.
20. Kaser SA, Fankhauser G, Glauser PMet al. Diagnostic value of inflammation markers in predicting perforation in acute sigmoid diverticulitis. World J Surg 2010;34(11):2717-22.
21. Biondo S, Borao JL, Millan Met al. Current status of the treatment of acute colonic diverticulitis: a systematic review. Colorectal Dis 2011.
22. Beckham H, Whitlow CB. The medical and nonoperative treatment of diverticulitis. Clin Colon Rectal Surg 2009;22(3):156-60.
23. Kircher MF, Rhea JT, Kihiczak Det al. Frequency, sensitivity, and specificity of individual signs of diverticulitis on thin-section helical CT with colonic contrast material: experience with 312 cases. AJR Am J Roentgenol 2002;178(6):1313-8.
24. Ambrosetti P, Grossholz M, Becker Cet al. Computed tomography in acute left colonic diverticulitis. Br J Surg 1997;84(4):532-4.
25. Werner A, Diehl SJ, Farag-Soliman Met al. Multi-slice spiral CT in routine diagnosis of suspected acute left-sided colonic diverticulitis: a prospective study of 120 patients. Eur Radiol 2003;13(12):2596-603.
26. Ripolles T, Agramunt M, Martinez MJet al. The role of ultrasound in the diagnosis, management and evolutive prognosis of acute left-sided colonic diverticulitis: a review of 208 patients. Eur Radiol 2003;13(12):2587-95.
27. Liljegren G, Chabok A, Wickbom Met al. Acute colonic diverticulitis: a systematic review of diagnostic accuracy. Colorectal Dis 2007;9(6):480-8.
28. Baker ME. Imaging and interventional techniques in acute left-sided diverticulitis. J Gastrointest Surg 2008;12(8):1314-7.
29. Ambrosetti P, Jenny A, Becker Cet al. Acute left colonic diverticulitis--compared performance of computed tomography and water-soluble contrast enema: prospective evaluation of 420 patients. Dis Colon Rectum 2000;43(10):1363-7.
30. Heverhagen JT, Sitter H, Zielke Aet al. Prospective evaluation of the value of magnetic resonance imaging in suspected acute sigmoid diverticulitis. Dis Colon Rectum 2008;51(12):1810-5.
31. Mizuki A, Nagata H, Tatemichi Met al. The out-patient management of patients with acute mild-to-moderate colonic diverticulitis. Aliment Pharmacol Ther 2005;21(7):889-97.
32. Etzioni DA, Chiu VY, Cannom RRet al. Outpatient treatment of acute diverticulitis: rates and predictors of failure. Dis Colon Rectum 2010;53(6):861-5.
33. Evans J, Kozol R, Frederick Wet al. Does a 48-hour rule predict outcomes in patients with acute sigmoid diverticulitis? J Gastrointest Surg 2008;12(3):577-82.
34. Rafferty J, Shellito P, Hyman NHet al. Practice parameters for sigmoid diverticulitis. Dis Colon Rectum 2006;49(7):939-44.
35. de Korte N, Unlu C, Boermeester MAet al. Use of antibiotics in uncomplicated diverticulitis. Br J Surg 2011;98(6):761-7.
36. de Korte N, Klarenbeek BR, Kuyvenhoven JPet al. Management of diverticulitis. Results of a survey among gastroenterologists and surgeons. Colorectal Dis 2011.
37. Brook I, Frazier EH. Aerobic and anaerobic microbiology in intra-abdominal infections associated with diverticulitis. J Med Microbiol 2000;49(9):827-30.
38. Kaiser AM, Jiang JK, Lake JPet al. The management of complicated diverticulitis and the role of computed tomography. Am J Gastroenterol 2005;100(4):910-7.
39. Kellum JM, Sugerman HJ, Coppa GFet al. Randomized, prospective comparison of cefoxitin and gentamicin-clindamycin in the treatment of acute colonic diverticulitis. Clin Ther 1992;14(3):376-84.
40. Ridgway PF, Latif A, Shabbir Jet al. Randomized controlled trial of oral vs intravenous therapy for the clinically diagnosed acute uncomplicated diverticulitis. Colorectal Dis 2009;11(9):941-6.
41. Schug-Pass C, Geers P, Hugel Oet al. Prospective randomized trial comparing short-term antibiotic therapy versus standard therapy for acute uncomplicated sigmoid diverticulitis. Int J Colorectal Dis 2010;25(6):751-9.
42. Klarenbeek BR, Bergamaschi R, Veenhof AAet al. Laparoscopic versus open sigmoid resection for diverticular disease: follow-up assessment of the randomized control Sigma trial. Surg Endosc 2011;25(4):1121-6.
43. Klarenbeek BR, Veenhof AA, Bergamaschi Ret al. Laparoscopic sigmoid resection for diverticulitis decreases major morbidity rates: a randomized control trial: short-term results of the Sigma Trial. Ann Surg 2009;249(1):39-44.
44. Jones OM, Stevenson AR, Clark Det al Laparoscopic resection for diverticular disease: follow-up of 500 consecutive patients. Ann Surg 2008;248(6):1092-7.
45. Leroy J, Cahill RA, Asakuma Met al. Single-access laparoscopic sigmoidectomy as definitive surgical management of prior diverticulitis in a human patient. Arch Surg 2009;144(2):173-9; discussion 79.
46. Thaler K, Baig MK, Berho Met al. Determinants of recurrence after sigmoid resection for uncomplicated diverticulitis. Dis Colon Rectum 2003;46(3):385-8.
47. Caselli G, Bambs C, Pinedo Get al. [Laparoscopic approach for intestinal passage reconstruction after Hartmann's operation: experience with 30 patients]. Cir Esp 2010;88(5):314-8.
48. Siddiqui MR, Sajid MS, Baig MK. Open vs laparoscopic approach for reversal of Hartmann's procedure: a systematic review. Colorectal Dis 2010;12(8):733-41.
49. Trenti L, Biondo S, Golda Tet al. Generalized peritonitis due to perforated diverticulitis: Hartmann's procedure or primary anastomosis? Int J Colorectal Dis 2011;26(3):377-84.
50. Biondo S, Perea MT, Rague JM et al. One-stage procedure in non-elective surgery for diverticular disease complications. Colorectal Dis 2001;3(1):42-5.
51. Khot UP, Lang AW, Murali Ket al. Systematic review of the efficacy and safety of colorectal stents. Br J Surg 2002;89(9):1096-102.
52. Small AJ, Young-Fadok TM, Baron TH. Expandable metal stent placement for benign colorectal obstruction: outcomes for 23 cases. Surg Endosc 2008;22(2):454-62.
53. Forshaw MJ, Sankararajah D, Stewart Met al. Self-expanding metallic stents in the treatment of benign colorectal disease: indications and outcomes. Colorectal Dis 2006;8(2):102-11.
54. Toorenvliet BR, Swank H, Schoones JW et al. Laparoscopic peritoneal lavage for perforated colonic diverticulitis: a systematic review. Colorectal Dis 2010;12(9):862-7.
55. Myers E, Hurley M, O'Sullivan GCet al. Laparoscopic peritoneal lavage for generalized peritonitis due to perforated diverticulitis. Br J Surg 2008;95(1):97-101.
56. Ambrosetti P, Chautems R, Soravia Cet al. Long-term outcome of mesocolic and pelvic diverticular abscesses of the left colon: a prospective study of 73 cases. Dis Colon Rectum 2005;48(4):787-91.
57. Machicado GA, Jensen DM. Acute and chronic management of lower gastrointestinal bleeding: cost-effective approaches. Gastroenterologist 1997;5(3):189-201.
58. Egger B, Peter MK, Candinas D. Persistent symptoms after elective sigmoid resection for diverticulitis. Dis Colon Rectum 2008;51(7):1044-8.
59. Ambrosetti P, Francis K, De Peyer Ret al Colorectal anastomotic stenosis after elective laparoscopic sigmoidectomy for diverticular disease: a prospective evaluation of 68 patients. Dis Colon Rectum 2008;51(9):1345-9.
60. Strate LL, Liu YL, Aldoori WHet al. Obesity increases the risks of diverticulitis and diverticular bleeding. Gastroenterology 2009;136(1):115-22 e1.
61. Aldoori WH, Giovannucci EL, Rimm EBet al. Use of acetaminophen and nonsteroidal anti-inflammatory drugs: a prospective study and the risk of symptomatic diverticular disease in men. Arch Fam Med 1998;7(3):255-60.
62. Strate LL, Liu YL, Aldoori WHet al. Physical activity decreases diverticular complications. Am J Gastroenterol 2009;104(5):1221-30.
63. Aldoori W, Ryan-Harshman M. Preventing diverticular disease. Review of recent evidence on high-fibre diets. Can Fam Physician 2002;48:1632-7.
64. Unlu C, Daniels L, Vrouenraets BCet al. A systematic review of high-fibre dietary therapy in diverticular disease. Int J Colorectal Dis 2011.
65. Strate LL, Liu YL, Syngal Set al. Nut, corn, and popcorn consumption and the incidence of diverticular disease. JAMA 2008;300(8):907-14.
66. Fric P, Zavoral M. The effect of non-pathogenic Escherichia coli in symptomatic uncomplicated diverticular disease of the colon. Eur J Gastroenterol Hepatol 2003;15(3):313-5.
67. Tursi A, Brandimarte G, Giorgetti GMet al. Mesalazine and/or Lactobacillus casei in maintaining long-term remission of symptomatic uncomplicated diverticular disease of the colon. Hepatogastroenterology 2008;55(84):916-20.
68. Colecchia A, Vestito A, Pasqui Fet al Efficacy of long term cyclic administration of the poorly absorbed antibiotic Rifaximin in symptomatic, uncomplicated colonic diverticular disease. World J Gastroenterol 2007;13(2):264-9.
69. Di Mario F, Aragona G, Leandro Get al. Efficacy of mesalazine in the treatment of symptomatic diverticular disease. Dig Dis Sci 2005;50(3):581-6.
70. Maconi G, Barbara G, Bosetti Cet al. Treatment of diverticular disease of the colon and prevention of acute diverticulitis: a systematic review. Dis Colon Rectum 2011;54(10):1326-38.
71. Schwesinger WH, Page CP, Gaskill HV, 3rdet al. Operative management of diverticular emergencies: strategies and outcomes. Arch Surg 2000;135(5):558-62; discussion 62-3.
72. Wasvary H, Turfah F, Kadro Oet al. Same hospitalization resection for acute diverticulitis. Am Surg 1999;65(7):632-5; discussion 36.
73. Sher ME, Agachan F, Bortul Met al. Laparoscopic surgery for diverticulitis. Surg Endosc 1997;11(3):264-7.

The above article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.




