Evaluation of the effect of magnesium vs. midazolam as adjunct to epidural bupivacaine in patients undergoing total knee replacement
Mohamed A. Daabiss and Abir Kandil
Cite this article as: BJMP 2013;6(2):a610
|
|
Abstract Background and objectives: Effective pain management is an important component of intraoperative and postsurgical care; it can prevent pain related clinical complications and improve the patient quality of life. This prospective, randomized, double-blind study was designed to evaluate analgesic efficacy of adding magnesium and midazolam to epidural bupivacaine in patients undergoing total knee replacement. |
Introduction
The effective relief of pain is of paramount importance to anyone treating patients undergoing surgery. Not only does effective pain relief mean a smoother postoperative course with earlier discharge from hospital, but it may also reduce the onset of chronic pain syndromes1. Regional anaesthesia is a safe, inexpensive technique, with the advantage of prolonged postoperative pain relief. Research continues concerning different techniques and drugs that could prolong the duration of regional anaesthesia and postoperative pain relief with minimal side effects1. Magnesium is the fourth most plentiful cation in the body. It has antinociceptive effects in animal and human models of pain 2,3. Previous studies had proved the efficacy of intrathecally administered magnesium in prolonging intrathecal opioid analgesia without increase in its side effectsThese effects have prompted the investigation of epidural magnesium as an adjuvant for postoperative analgesia4.
Midazolam, a water-soluble benzodiazepine, has proved epidural analgesic effect in patients with postoperative wound painSerum concentrations of midazolam after an epidural administration were smaller than those producing sedative effects in humans5.
The purpose of this study is to compare the analgesic efficacy of epidural magnesium to that of midazolam when administered with bupavacaine in patients undergoing total knee replacement.
Methods:
After obtaining the approval of the Hospital Research & Ethical Committee and patient’s informed consent, 120 ASA I and II patients of both sexes, aged 50-70 years undergoing total knee replacement surgery were enrolled in this randomised, double blinded placebo-controlled study. Those who had renal, hepatic impairment, cardiac disease, spine deformity, neuropathy, coagulopathy or receiving anticoagulants for any cause were excluded from the study.
Prior to surgery, the epidural technique as well as the visual analogue scale (VAS; 0: no pain; 10: worst pain) and the patient-controlled epidural analgesia device (PCEA) were explained to the patients.
The protocol was similar for all patients. Patients received no premedication. Heart rate (HR), mean arterial pressure (MAP) and oxygen saturation (SpO2) were measured. Intravenous access had been established and an infusion of crystalloid commenced.
Before the induction of anaesthesia, an epidural catheter was placed at the L3-L4 or L4-L5 intervertebral space under local anaesthesia with the use of loss of resistance technique, and correct position was confirmed by injection of lidocaine 2% (3ml) with epinephrine in concentration 1: 200 000. An epidural catheter was then inserted into the epidural space. The level to be blocked was up to TIn a double blind fashion and using a sealed envelope technique, patients were randomly allocated to one of three equal groups to receive via epidural catheter either 50 mg magnesium sulphate (MgSO4) in 10 ml as an initial bolus dose followed by infusion of 10 mg/h (diluted in 10 ml saline) during the surgery (Mg group) or 10 ml saline followed by infusion of saline 10 ml/h during the surgery (control group) or 0.05 mg/kg of midazolam in 10 ml saline (Midazolam group) followed by infusion of saline 10 ml/h during the surgery. All patients received epidural bupivacaine 0.5 % in a dose of 1ml/segment .
Sensory block was assessed bilaterally by using loss of temperature sensation with an ice cube. Motor block was evaluated using a modified Bromage scale 6 (0: no motor block, 1: inability to raise extended legs, 2: inability to flex knees, 3: inability to flex ankle joints). During the course of operation, epidural bupivacaine 0.5% was given, if required, to achieve a block above T10MAP, HR, SpO2 and respiratory rate (RR) were recorded before and after administration of the epidural medications and every 5 minutes till end of the surgery.
When surgery was complete, all patients received PCEA using a PCEA device (Infusomat® Space, B.Braun Space, Germany) containing fentanyl 2 µg/ml and bupivacaine 0.08% (0.8 mg/ml). The PCEA was programmed to administer a demand bolus dose of fentanyl 5 ml with no background infusion and lockout interval 20 min. The PCEA bolus volume was titrated according to analgesic effect or occurrence of side-effects. Patients’ first analgesic requirement times were recorded. The time from the completion of the surgery until the time to first use of rescue medication by PCEA was defined as the time to first requirement for postoperative epidural analgesia. A resting pain score of ≤ 3 was considered as a satisfactory pain relief. If patients had inadequate analgesia, supplementary rescue analgesia with intramuscular pethidine 50 mg was available. MAP, HR, SpO2, RR and pain assessment using VAS were recorded at 30 minutes, and then at 1, 2, 4, 8, 12, and 24 h in the postoperative period. Epidural fentanyl consumption was also recorded at the same time points. Patients were discharged to the ward when all hemodynamic variables were stable with completely resolved motor block, satisfactory pain relief, and absence of nausea and vomiting. Adverse events related with the epidural drugs (sedation, respiratory depression, nausea, vomiting, prolonged motor block) and epidural catheter were recorded throughout the 24 h study period. Sedation was assessed with a five-point Scale: 1: Alert/active, 2: Upset/wary, 3: Relaxed, 4: Drowsy, 5: Asleep. A blinded anaesthesiologist who was unaware of the drug given, performed all assessments.
The results were analyzed using SPSS version 17. The number of subjects enrolled was based on a power calculation of finding a 20% change in HR and MAP. The α-error was assumed to be 0.05 and the type II error was set at 0.20. Numerical data are presented as median and 95% CI. The groups were compared with analysis of variances (ANOVA). The VAS pain scores were analyzed by Mann-Whitney U test. Categorical data were compared using the Chi square test. P value of 0.05 was used as the level of significance.
Results:
The three groups were comparable in respect of age, weight, height, sex, ASA status and duration of surgery (Table 1). Patients in all groups were comparable regarding intra or postoperative MAP, HR (Figure 1,2), RR and SpO2 during the observation period with no case of hemodynamic or respiratory instability. No difference in the quality of sensory and motor block before and during the surgery was noted between groups, and none of the patients required supplemental analgesia during surgery.
| Control | Mg | Midazolam | |
| of patients | 40 | 40 | 40 |
| Sex (female/male) | 17/23 | 20/20 | 19/21 |
| Age (yrs) | 59.5 ± 6.1 | 61.1 ± 4.9 | 61.9 ± 3 |
| ASA (I/II) | 12/28 | 14/26 | 11/29 |
| Weight (Kg) | 69.7 ± 4.2 | 66.9 ± 6.7 | 70.1 ± 5.5 |
| Height (cm) | 165.9 ± 8.6 | 170.2 ± 4.5 | 167.2 ± 6.9 |
| Duration of surgery (min) | 144 ± 21 | 129 ± 30 | 130 ± 27 |
|
( median and 95% CI or number). No significant difference among groups |
|||
Table 1: Demographic data and duration of surgery.
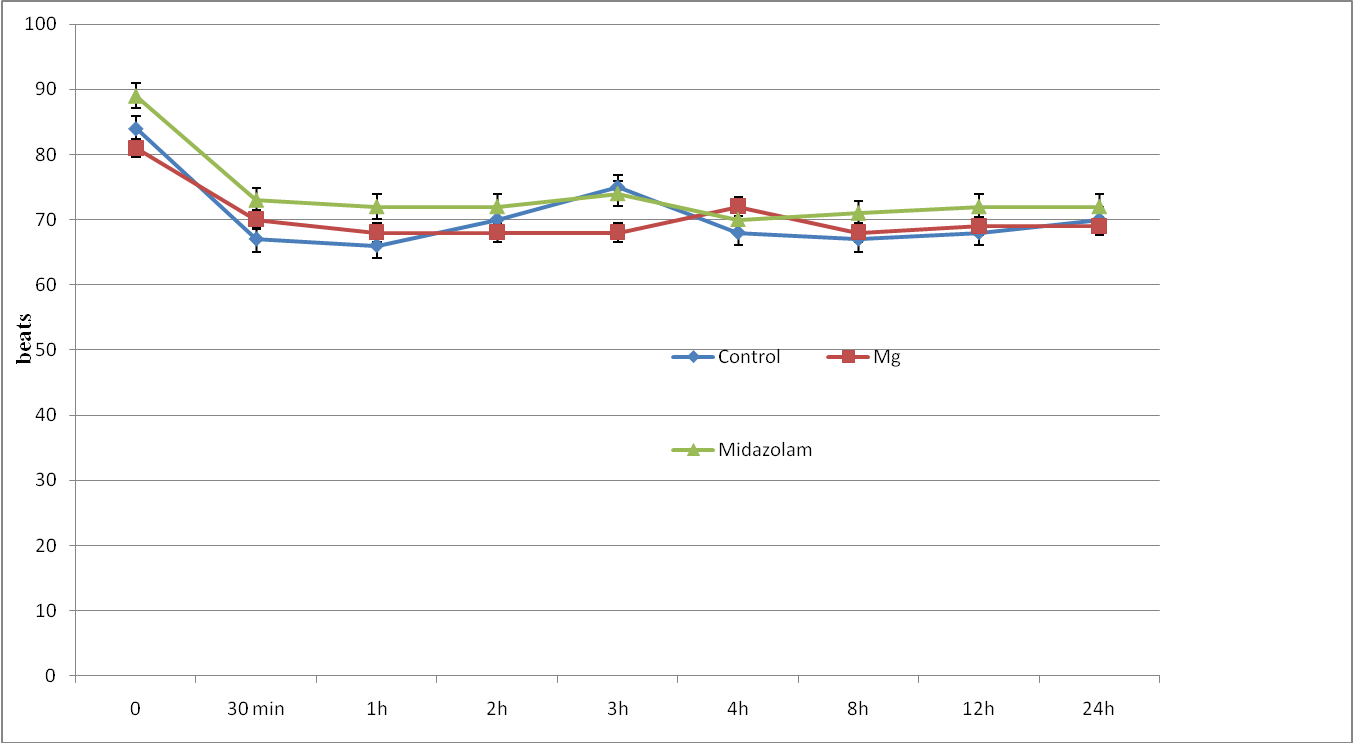
Figure 1: Heart rate changes (HR) of study groups. Data are mean±SD.
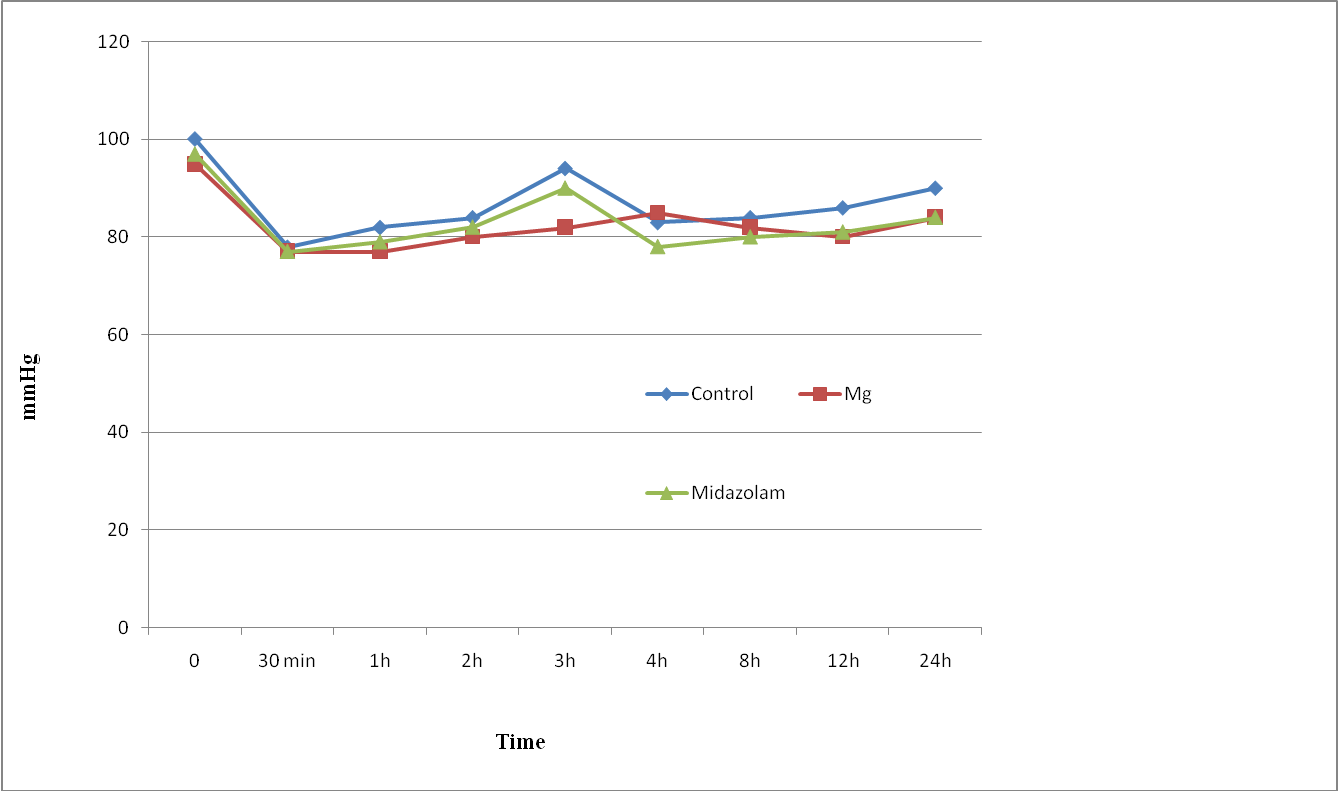
Figure 2: Mean Arterial pressure changes (MAP) of study groups. Data are mean±SD.
The intraoperative VAS was significantly less in magnesium and midazolam groups compared to control group after 15 and 30 minutes (Figure 3). Whereas the postoperative VAS was significantly less in the magnesium group in the first postoperative hour compared to other groups (Figure 4).
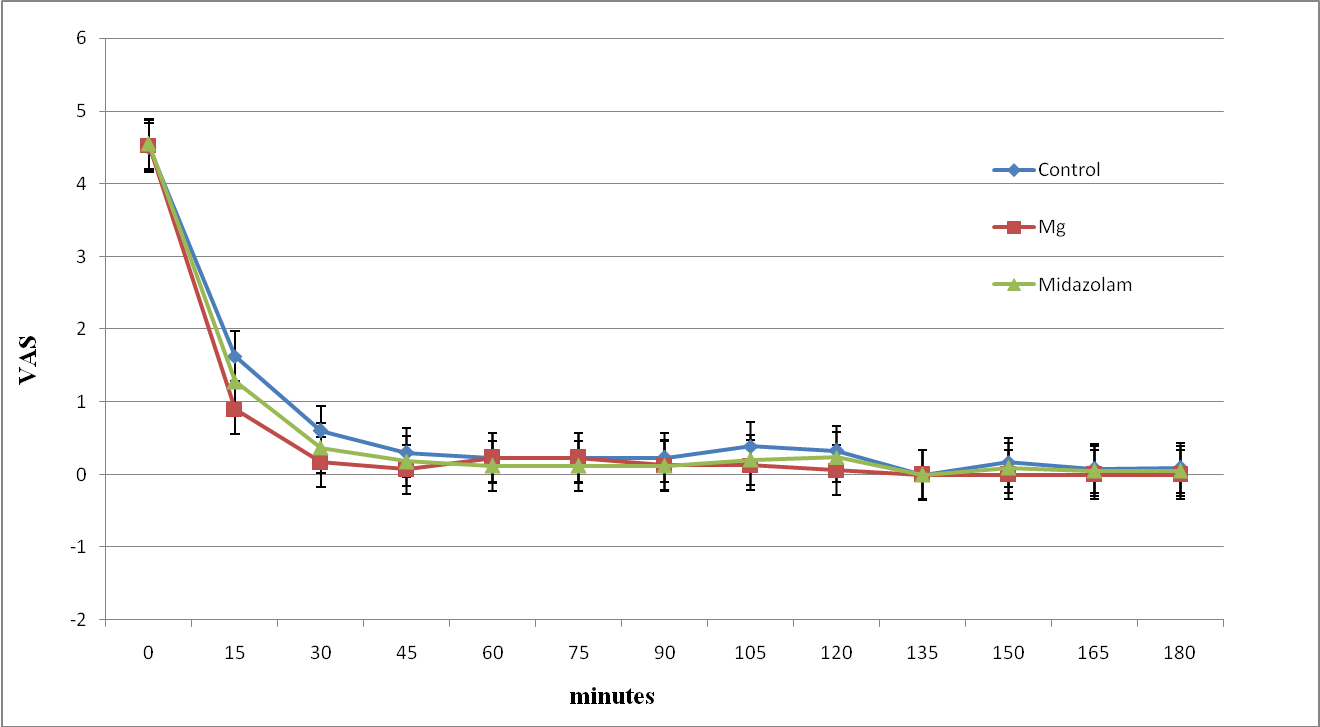
Figure 3: The intra-operative Visual analogue score of study groups. Data are mean±SD.

Figure 4: The post-operative Visual analogue score of study groups. Data are mean±SD.
The time of request for postoperative analgesia was significantly delayed and the number of patients requesting postoperative analgesia was significantly reduced in magnesium group (Figure 5). Moreover, the pethidine rescue analgesia consumption and the total amount of postoperative fentanyl infusion were significantly reduced in magnesium group compared to other groups (Table 2) (Figure 5).
| Control | Mg | Midazolam | P | |
| Pethidine (mg) | 92.38±10.91 | 52.56±9.67 | 70±9.23 | 0.014* |
| Total Fentanyl infusion (mcg)/24H | 320.67±112.19 | 219.9±56.86 | 256.2±53.49 | 0.00* |
|
Data are expressed as median and 95% CI. * Significant difference (P < 0.05). |
||||
Table 2: Pethidine rescue analgesia and total fentanyl infusion over 24 hours of study groups

Figure 5: The number of patients and time of requesting analgesia in the first 3 postoperative hours in the study groups. Data are numbers.
No significant differences were recorded regarding the incidence of sedation or any adverse effects between groups (Table 3).
| Control | Mg | Midazolam | P | |
| Sedation | 0 | 0 | 2 | 0.068 |
| Bradycardia | 1 | 0 | 0 | 0.103 |
| Nausea & Vomiting | 3 | 1 | 2 | 0.571 |
|
Data are expressed as numbers. Significant difference (P < 0.05). |
||||
Table 3: Incidence of sedation, bradycardia and nausea & vomiting in the study groups
Discussion:
The efficacy of postoperative pain therapy is a major issue in the functional outcome of the surgery7. It was evident that epidural analgesia regardless the agent used provides better postoperative analgesia compared with parental analgesiaThe addition of adjuvants to local anaesthetics in epidural analgesia gained widespread popularity as it provides a significant analgesia which allows the reduction of the amount of local anaesthetic and opioid administration for postoperative pain and thus the incidence of side effects9.
Our study demonstrates a significant intraoperative improvement in VAS in magnesium and midazolam groups, while in the postoperative period magnesium group showed a significant reduction in the number of patients requesting early postoperative analgesia as well as total fentanyl consumption.
The antinociceptive effects of magnesium are primarily based on the regulation of calcium influx into the cell, as a calcium antagonism and antagonism of N-methyl-D-aspartate (NMDA) receptorTanmoy and colleagues10 evaluated the effect of adding MgSO4 as adjuvants to epidural Bupivacaine in lower abdominal surgery and reported reduction in time of onset and establishment of epidural block. Whereas, Arcioni and colleagues 11 proved that combined intrathecal and epidural MgSO4 supplementation reduce the postoperative analgesic requirements. Farouk et al12 found that the continuous epidural magnesium started before anesthesia provided preemptive analgesia, and analgesic sparing effect that improved postoperative analgesia. Also, Bilir and colleagues 4 showed that the time to first analgesia requirement was slightly longer with significant reduction in fentanyl consumption after starting epidural MgSO4 infusion postoperatively. Asokumar and colleagues13 found that addition of MgSO4 prolonged the median duration of analgesia after intrathecal drug administration.
On the other hand, Ko and colleagues14 found that peri-operative intravenous administration of magnesium sulfate 50 mg/kg does not reduce postoperative analgesic requirements which could be attributed to the finding that the perioperative intravenous administration of MgSO4 did not increase CSF magnesium concentration due to inability to cross blood brain barrier.
Nishiyama et al17,18,19 reported that epidural midazolam was useful for postoperative pain relief. It was suggested that epidurally administered midazolam exerts its analgesic effects through the ᵞ-aminobutyric acid receptors in the spinal cord, particularly in lamina II of the dorsal horn15 as well as through the opioid receptorsNishiyama et al20 showed that intrathecally administered midazolam and bupivacaine had synergistic analgesic effects on acute thermal- or inflammatory-induced pain, with decreased behavioral side effects. While, Kumar et al21 reported that single-shot caudal coadminstration of bupivacaine with midazolam 50 µg/kg was associated with extended duration of postoperative pain relief in lower abdominal surgery. Whereas, Jaiswal et al22 concluded that epidural midazolam can be useful and safe adjunct to bupivacaine used for epidural analgesia during labor.
In the present study, there were no significant hemodynamic changes between groups. This is in agreement with many authors who used epidural MgSO44,12,23 and midazolam 24 and did not report any hemodynamic or respiratory instability during the observation period.
This study did not record any neurological or epidural drugs related complications postoperatively. Our results are in accord with some of the trials that have previously examined the neurological complications of using epidural MgSO4,11,12,23. Moreover, Goodman and colleagues 25, found that inadvertent administration of larger doses MgSO4 (8.7 g and 9.6 g) through epidural catheter did not reveal any neurological side effects.
Regarding epidural midazolam, Nishiyama19 said that epidural administration of midazolam has a wide safety margin for neurotoxicity of the spinal cord due to the small dose used.
Our results did not reveal any significant difference regarding the sedation score. This is in agreement with Bilir et al4 and El-Kerdawy23 who did not report any case with drowsiness or respiratory depression when using epidural magnesium.
Whereas, De Beer et al26 and Nishiyama et al27 reported that a dose of 50 µg/kg midazolam appears to be the optimum dose for epidural administration, while many patients fell into complete sleep with no response to verbal command and respiratory depression when they used epidural midazolam 0.075 mg/Kg or 0.01 mg/KgMoreover, Nishiyama et al17,28 reported that when 50 µg/kg epidural midazolam was used, serum midazolam concentration was less than 200 ng/ml which was considered as the lower limit for sedation by intravenous administration.
In conclusion, co-administration of epidural magnesium provides better intraoperative analgesia as well as analgesic-sparing effect on PCEA consumption without increasing the incidence of side-effects compared to bupivacaine alone or with co-administration of epidural midazolam in patients undergoing total knee replacement. The results of the present investigation suggest that magnesium may be one of the useful adjuvants to epidural analgesia.
|
Competing Interests None declared Author Details MOHAMED A DAABISS, MD, Department of Anaesthesia, Riyadh Armed Forces Hospital, Saudi Arabia. ABIR KANDIL, MS, Department of Anaesthesia, Riyadh Armed Forces Hospital, Saudi Arabia. CORRESPONDENCE: DR MOHAMED A DAABISS, Department of Anaesthesia, Riyadh Armed Forces Hospital, Saudi Arabia. Email: madaabiss@yahoo.com |
References
1.Sirvinskas E, Laurinaitis R. Use of magnesium sulfate in anesthesiology. Medicine 2002; 38: 147–50
2.Begon S, Pickering G, Eschalier A, Dubray C. Magnesium increases morphine analgesic effect in different experimental models of pain. Anesthesiol 2002; 96: 627–32.
3.Kroin JS, McCarthy RJ, Von Roenn N, Schwab B, Tuman KJ, Ivankovich AD. Magnesium sulfate potentiates morphine antinociception at the spinal level. Anesth Analg 2000; 90: 913–7.
4.Bilir A, Gulec S, Erkan A, Ozcelik A. Epidural magnesium reduces postoperative analgesic requirement. Br J Anaesth 2007; 98: 519-23.
5.Nishiyama T, Tamai H, Hanaoka K. Serum and Cerebrospinal Fluid Concentrations of Midazolam After Epidural Administration in Dogs. Anesth Analg 2003;96:159 –62.
6.Bromage PR. A comparison of the hydrochloride and carbon dioxide salts of lidocaine and prilocaine in epidural analgesia. Acta Anesthesiol Scand Supple 1965; 75: 193–200.
7.Shaved Y, Berlin B, Trade-in E, Boris M. The effects of postoperative pain management on immune response to surgery. Anesth Analg 2003; 97: 822-7.
8.Brian M, Spencer S, Liu R, Anne R, Cowan A, John A, et al. Efficacy of postoperative epidural analgesia, a meta analysis. JAMA 2003; 290(18): 2455-64.
9.Whalen BM, Roewer N, Kranke P. Use of local anaesthetics and adjuncts for spinal and epidural anaesthesia and analgesia at German and Austerian university. Anesthesiol 2010, 10:4.
10.Tanmoy G, Chandra G, Malik A, Singh D, Bhatia V. Evaluation of the effect of magnesium sulphate vs. Midazolam as adjunct to epidural bupivacaine. Indian J Anesth 2010;54:308-13.
11.Arcioni R, Palmisani S, Tigano S, Santorsola C, Sauli V, Romano S, et al. Combined intrathecal and epidural magnesium sulfate supplementation of spinal anesthesia to reduce postoperative analgesic requirements. Acta Anaesthesiol Scand 2007; 51:482-9.
12.Farouk S, Ibrahim S. Pre-incisional epidural magnesium provides pre-emptive and preventive analgesia in patients undergoing abdominal hysterectomy. Br J Anaesth 2008; 101: 694-9.
13.Asokumar B, McCarthy RJ, Kroin JS, Leon W, Perry p, Tuman KJ. Intrathecal Magnesium prolongs fentanyl analgesia. Anesth Analg 2002; 95: 661-6.
14.Ko SH, Lim HR, Kim DC, Han YJ, Choe H, Song HS. Magnesium sulfate does not reduce postoperative analgesic requirements. Anesthesiol 2001; 95(3): 640-6.
15.Edwards M, Serrao JM, Gent JP, et al. On the mechanism by which midazolam causes spinally mediated analgesia. Anesthesiol 1990; 73: 273-7.
16.Serrao JM, Goodchild CS, Gent JP. Reversal by naloxone of spinal antinociceptive effects of fentanyl, ketocyclazocine, and midazolam. Eur J Anaesthesiol 1991;8: 401– 6.
17.Nishiyama T, Odaka Y, Hirasaki A, seto K. Epidural midazolam for treatment of postoperative pain. Masui 1991;40(9):1353-8.
18.Nishiyama T, Matsukawa T, Hanaoka K. Continuous epidural administration of midazolam and bupivacaine for postoperative analgesia. Acta Anaesthesiol Scand 1999; 43 (5): 568–72.
19.Nishiyama T. The post-operative analgesic action of midazolam following epidural administration. Eur J Anaesthesiol 1995; 12 : 369-74.
20.Nishiyama T, Hanaoka K. Midazolam Can Potentiate the Analgesic Effects of Intrathecal Bupivacaine on Thermal- or Inflammatory-Induced Pain. Anesth Analg 2003;96:1386 –91.
21.Kumar P, Rudra A, Pan AK, Acharya A. Caudal Additives in Pediatrics: A Comparison Among Midazolam, Ketamine, and Neostigmine Coadministered with Bupivacaine. Anesth Analg 2005;101:69 –73.
22.Jaiswal S, Ranjan P, Tewari N, Agarwal NR, Mathur SK. Comparative Study Of Epidural Midazolam And Butorphanol As Adjuvant With Bupivacaine For Labor Analgesia: A Double Blind Study. Internet J Anesthesiol 2007;14(1).
23.El-Kerdawy H. Analgesic requirements for patients undergoing lower extremity orthopedic surgery, the effect of combined spinal and epidural magnesium. Middle East J Anesth 2008;19(5):1013-26.
24.Nishiyama T, Yokoyama T, Hanaoka K. Midazolam improves postoperative epidural analgesia with continuous infusion of local anaesthetics. Can J Anaesth 2008;45(6): 551-5.
25.Goodman EJ, Haas AJ, Kantor GS. Inadvertent administration of magnesium sulphate through epidural catheter: report and analysis of a drug error. Obst Anesth Digest 2006; 4: 199-200.
26.De Beer DAH, Thomas ML. Caudal additives in children: solutions or problems? Br J Anaesth 2003;90:487–98.
27.Nishiyama T, Hirasaki A, Odaka Y, Konishi H, Seto K, Goto I. Epidural midazolam with saline, optimal dose for postoperative pain. Masui.1992;41(1):49-54.
28.Nishiyama T, Hanaoka K. Effect of diluents volume on post- operative analgesia and sedation produced by epidurally administered midazolam. Eur J Anaesthesiol 1998;15:275–9.

The above article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.




