Effectiveness of Chlorhexidine oral decontamination in reducing the incidence of ventilator associated pneumonia: A meta-analysis.
E Balamurugan, A Kanimozhi and Govinda Kumari
Cite this article as: BJMP 2012;5(1):a512
|
|
Abstract Background and Purpose: Ventilator-associated pneumonia (VAP) is an important nosocomial infection worldwide, which leads to increased length of hospital stay, healthcare costs and mortality. Evidence on oral decontamination with antiseptic in reducing VAP is limited. Hence, a meta-analysis was performed to determine the effect of chlorhexidine oral decontamination in the reduction of VAP in mechanically ventilated patients Methods: An extensive literaturereview was conducted using the following databases: CINAHL, MEDLINE, Joanna Briggs Institute, Cochrane Library, EMBASE, CENTRAL, and the Google search engine. Retrieved articles were selected based on the methodological quality, inclusion criteria and analysed to find the pooled effect size. Results: The nine trials included in this meta-analysis revealed a significant reduction in the incidence of VAP among patients who received prophylactic oral decontamination with Chlorhexidine. However no significant effect was found in reducing overall mortality rate among the mechanically ventilated patients. Conclusion: The safety profile regarding the possible selection and induction of antibiotic resistance and presumed cost benefits of Chlorhexidine make it a highly attractive intervention for the prevention of VAP. This meta-analysis indicated that chlorhexidine can serve as a cost-effective and safe antiseptic in preventing VAP in mechanically ventilated patients. Keywords: Chlorhexidine; Oral decontamination; Ventilator associated pneumonia; Mechanical ventilation |
Introduction
Nosocomial pneumonia in patients receiving mechanical ventilation, also called ventilator-associated pneumonia (VAP), is an important nosocomial infection worldwide which leads to an increased length of hospital stay, healthcare costs, and mortality.(1,2,3,4,5) The incidence of VAP ranges from 9% to 27% with a crude mortality rate that can exceed up to 50%. (6,7,8,9) Aspiration of bacteria from the upper digestive tract is an important proposed mechanism in the pathogenesis of VAP.(9, 10) The normal flora of the oral cavity may include up to 350 different bacterial species, with tendencies for groups of bacteria to colonize different surfaces in the mouth. For example, Streptococcus mutans, Streptococcus sanguis, Actinomyces viscosus, and Bacteroides gingivalis mainly colonize the teeth; Streptococcus salivarius mainly colonizes the dorsal aspect of the tongue; and Streptococcus mitis is found on both buccal and tooth surfaces.(11) Because of a number of processes, however, critically ill patients lose a protective substance called fibronectin from the tooth surface. Loss of fibronectin reduces the host defence mechanism mediated by reticuloendothelial cells. This reduction in turn results in an environment conducive to attachment of microorganism to buccal and pharyngeal epithelial cells.(12) Addressing the formation of dental plaque and its continued existence by optimizing oral hygiene in critically ill patients is an important strategy for minimizing VAP.(13) Two different interventions aimed at decreasing the oral bacterial load are selective decontamination of the digestive tract involving administration of non absorbable antibiotics by mouth, through a naso-gastric tube, and oral decontamination, which is limited to topical oral application of antibiotics or antiseptics.(14) Though meta-analysis of antibiotics in decontamination of digestive tracts have found positive results(15) , the use of this intervention is, however, limited by concern about the emergence of antibiotic resistant bacteria.(16) One alternative to oral decontamination with antibiotics is to use antiseptics, such as chlorhexidine which act rapidly at multiple target sites and accordingly may be less prone to induce drug resistance.(17) Recently a meta-analysis of four trials on chlorhexidine failed to show a significant reduction in rates of ventilator associated pneumonia(18) but, subsequent randomised controlled trials, however, suggested benefit from this approach.(19) Current guidelines from the Centres for Disease Control and Prevention recommend topical oral chlorhexidine 0.12% during the perioperative period for adults undergoing cardiac surgery (grade II evidence). The routine use of antiseptic oral decontamination for the prevention of ventilator associated pneumonia, however, remains unresolved.(8) Despite the lack of firm evidence favouring this preventive intervention, a recent survey across 59 European intensive care units from five countries showed that 61% of the respondents used oral decontamination with chlorhexidine. As the emphasis on evidence based practice is increasing day by day, integrating recent evidence by meta-analysis could greatly benefit patient care and ensure safer practices. Hence we carried out this meta-analytic review to ascertain the effect of oral decontamination using chlorhexidine in the incidence of ventilator associated pneumonia and mortality in mechanically ventilated adults.(20)
Methods
Articles published from 1990 to May 2011 in English which were indexed in the following databases were searched: CINAHL, MEDLINE, Joanna Briggs Institute, Cochrane Library, EMBASE, CENTRAL, and Google search engine. We also screened previous meta-analyses and the references lists from all the retrieved articles for additional studies. Further searches were carried out in two trial registers (www.clinicaltrials.gov/ and www.controlled-trials.com/) and on web postings from conference proceedings, abstracts, and poster presentations.
Articles retrieved were assessed for inclusion criteria by three independent reviewers from the field of nursing with masters degrees. The inclusion criteria set for this meta-analysis were as follows:
a) VAP definition meeting both clinical and radiological criteria
b) Intubation for more than 48 hours in ICU.
We excluded the studies where clinical pulmonary infection score alone was considered for diagnosing VAP. Thereafter the articles were evaluated for randomisation, allocation concealment, blinding techniques, clarity of inclusion and exclusion criteria, outcome definitions, similarity of baseline characteristics, and completeness of follow-up. We considered randomisation to be true if the allocation sequence was generated using computer programs, random number tables, or random drawing from opaque envelopes. Finally, based on the above characteristics, only 9 trials which fulfilled the inclusion criteria was included for the pooled analysis. A brief summary of the 9 trials were listed in Table 1. The primary outcomes in this meta-analysis were incidence of VAP and mortality rate.
Table 1: Brief summary of trials
| Source | Subjects | Intervention | Compared With | Outcome with respect to VAP | Outcome with respect to Mortality | ||||
| C | E | C | E | ||||||
| DeRiso et al., 1996 | 353- Open Heart surgery patients | Chlorhexidine 0.12% 15 ml preoperatively and twice daily postoperatively until discharge from intensive care unit or death | Placebo | 9/180 | 3/173 | 10/180 | 2/173 | ||
| Fourrier et al., 2000 | 60- Medical and surgical patients | Chlorhexidine gel 0.2% dental plaque decontamination 3 times daily, compared with bicarbonate solution rinse 4 times daily followed by oropharyngeal suctioning until 28 days discharge form ICU or death | Standard treatment | 15/30 | 5/30 | 7/30 | 3/30 | ||
| Houston et al., 2002 | 561- cardiac surgery patients | Chlorhexidine 0.12% rinse compared with Listerine preoperatively and twice daily for 10 days postoperatively or until extubation, tracheostomy, death, or diagnosis of pneumonia. | Standard treatment | 9/291 | 4/270 | NA | NA | ||
| MacNaughton et al., 2004 | 194 – Medical and surgical patients | Chlorhexidine 0.2% oral rinse twice daily until extubation or death | Placebo | 21/101 | 21/93 | 29/93 | 29/101 | ||
| Fourrier et al., 2005 | 228 –ICU patients | Chlorhexidine 0.2% gel three times daily during stay in intensive care unit until 28 days | Placebo | 12/114 | 13/114 | 24/114 | 31/114 | ||
| Segers et al., 2005 | 954 – cardiac surgery patients | Chlorhexidine 0.12%, nasal ointment, and 10 ml oropharynx rinse four times daily on allocation and admission to hospital until extubation or removal of nasogastric tube | Placebo | 67/469 | 35/485 | 6/469 | 8/485 | ||
| Boop et al., 2006 | 5- cardiac surgery patients as pilot study | 0.12% chlorhexidine gluconate oral care twice daily until discharge | Standard treatment | 1/3 | 0/2 | NA | NA | ||
| Koeman et al., 2006 | 385 –General ICU patients | 2 treatment group: 2%Chlorhexidine, chlorhexidine and colistin, placebo four times daily until diagnosis of ventilator associated pneumonia, death, or extubation | Placebo | 23/130 | 13/127 | 39/130 | 49/127 | ||
Tontipong et al., 2008 |
207 –General medical ICU or wards | 2% chlorhexidine solution times per day until endotracheal tubes were removed. | Standard treatment | 12/105 | 5/102 | 37/105 |
36/102 |
||
NA-Not available; C-Control group; E- Experimental group
Data analysis
Meta-analysis was performed in this study by using Review Manager 4.2 (Cochrane Collaboration, Oxford) with a random effect model. The pooled effects estimates for binary variables were expressed as a relative risk with 95% confidence interval. Differences in estimates of intervention between the treatment and control groups for each hypothesis were tested using a two sided z test. We calculated the number of patients needed to treat (NNT, with 95% confidence interval) to prevent one episode of ventilator associated pneumonia during the period of mechanical ventilation. A chi-squared test was used to assess the heterogeneity of the results. A Forest plot graph was drawn using Stats direct software version 2.72 (England: Stats Direct Ltd. 2008). We considered a two tailed P value of less than 0.05 as significant throughout the study.
Results
Effect of Chlorhexidine in reducing the Incidence of VAP
A total of nine trials were included in this meta-analysis(19,21,22,23,24,25,26,27,28). Pooled analysis of the nine trials with 2819 patients revealed a significant reduction in the incidence of VAP using chlorhexidine (Relative risk 0.60, 0.47 to 0.76; P< 0.01) (Figure 1). In relation to the Number Needed to Treat (NNT), 21 patients would need to receive oral decontamination with Chlorhexidine to prevent one episode of Ventilator associated pneumonia (NNT 21, 14 to 38).
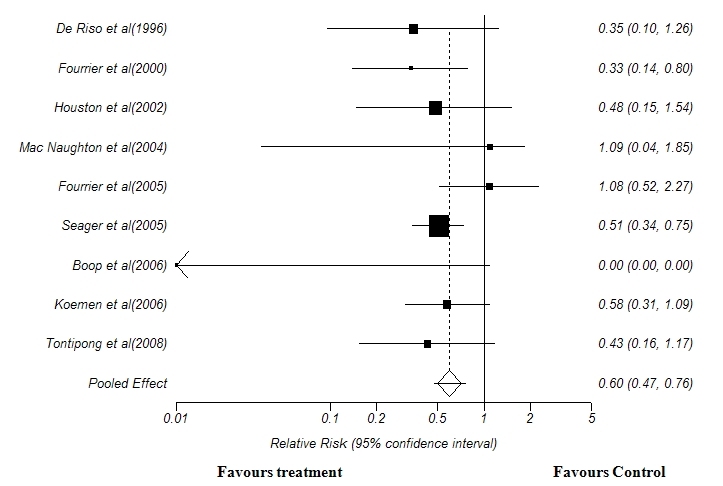
Figure 1: Forest Plot showing the effect of Chlorhexidine oral decontamination in preventing the incidence of ventilator-associated pneumonia. Test for heterogeneity:χ2 =15.5, df =8, p < 0.01. Test for overall effect: z =4.33, p <0.05.
Effect of Chorhexidine in overall mortality rate
For assessing the outcomes in terms of mortality, only seven out of nine trials were included, since the other two(23,27) did not report the mortality rate. Pooled analysis of the seven trials with 2253 patients revealed no significant effect in reducing the overall mortality rate in patient who received chlorhexidine oral decontamination.(Relative risk 1.02, 0.83 to 1.26; P= 0.781 (Figure 2).
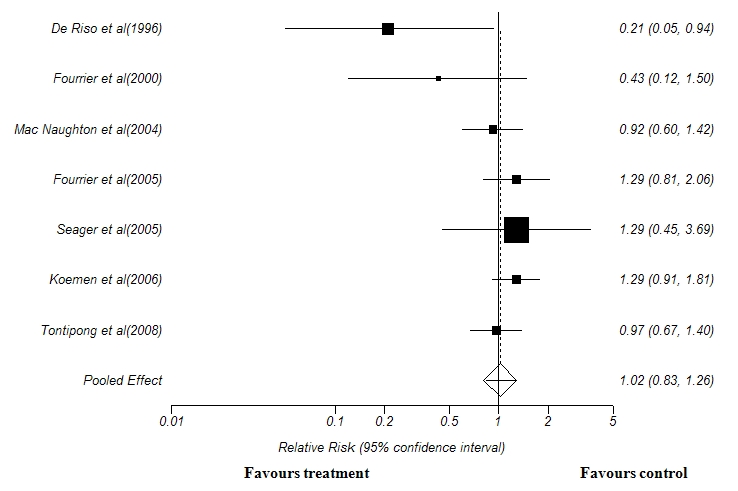
Figure 2: Forest plot showing the effect of Chlorhexidine oral decontamination in reducing overall mortality rate. Test for heterogeneity:χ2 =0.05, df =6, p = 0.81. Test for overall effect: z =0.27, p = 0.78
Discussion
The effectiveness of oral decontamination to prevent VAP in patients undergoing mechanical ventilation has remained controversial since its introduction, due to partly discordant results of individual trials. In the present meta-analysis nine trials were included to estimate the pooled effect size; the results revealed a significant reduction in the incidence of VAP among patients who were treated with oral chlorhexidine. But, it had no effect in reducing the overall mortality rate among these patients. There is a firm body of evidence that oropharyngeal colonization is pivotal in the pathogenesis of VAP. More than 25 years ago, Johanson et al described associations between increasing severity of illness, higher occurrence of oropharyngeal colonization, and an increased risk of developing VAP .(29,30)Subsequently, cohort and sequential colonization analyses identified oropharyngeal colonization as a important risk factor for VAP. (31,32,33) Our finding confirms the pivotal role of Oro- pharyngeal colonization in the pathogenesis of VAP , since this meta-analysis indicates that oral decontamination may reduce the incidence of VAP. Chlorhexidine was proven to have excellent antibacterial effects, with low antibiotic resistance rates seen in nosocomial pathogens, despite long-term use(34). Previous meta-analyses examining the effect of prophylaxis using selective decontamination of the digestive tract reported a significant reduction in the incidence of ventilator associated pneumonia(35,36,37). The most recent meta-analysis indicated that such an intervention combined with prophylactic intravenous antibiotics reduces overall mortality(38). In comparison our review suggests that oral antiseptic prophylaxis alone can significantly reduce the incidence of ventilator associated pneumonia, but not mortality. A similar result was documented by Ee Yuee Chan et al (2007)(14) who performed a meta-analysis with seven trials with a total of 2144 patients and found a significant result (Odds ratio 0.56, 0.39 to 0.81). Another comparable finding in the present study was, Mortality rate was not influenced by use of Chlorhexidine use, which was in line with the findings of Ee Yuee Chan et al (2007)(14) . Our meta-analysis on Chorhexidine differs from the findings of Pineda et al, who pooled four trials on chlorhexidine and did not report lower rates of ventilator associated pneumonia (odds ratio 0.42, 0.16-1.06; P=0.07)(18) . Our results also extend those of Chlebicki et al, who did not find a statistically significant benefit using the more conservative random effects model after pooling seven trials on chlorhexidine (relative risk 0.70, 0.47- 1.04; P=0.07), although their results were significant with the fixed effects model(39). Our meta-analysis included larger data set with a total of 9 trials including recent trials(28) which further adds strength to our analysis.
Limitations
Though our literature search was comprehensive, it is possible that we missed other relevant trials. Electronic and hand searches do not completely reflect the extent of research outcomes. For example, trials reported at conferences are more likely than trials published in journals to contain negative reports. In addition, more positive than negative results tend to be reported in the literature. This failure to publish more studies with negative outcomes is probably more due to authors’ lack of inclination to submit such manuscripts than to the unwillingness of editors to accept such manuscripts. Furthermore, many studies not published in English were not included e.g. a study by Zamora Zamora F (2011).(40) These limitations may lead to a risk for systematic reviews to yield a less balanced analysis and may therefore affect the recommendations resulting from the reviews. In addition, the heterogeneity which we found among the trials with respect to populations enrolled, regimens used, outcome definitions, and analysis strategies, may limit the ability to generalize results to specific populations.
Conclusion
The finding that chlorhexidine oral decontamination can reduce the incidence of ventilator associated pneumonia could have important implications for lower healthcare costs and a reduced risk of antibiotic resistance compared with the use of antibiotics. These results should be interpreted in light of the moderate heterogeneity of individual trial results and possible publication bias. It may not be prudent to adopt this practice routinely for all critically ill patients until strong data on the long term risk of selecting antiseptic and antibiotic resistant organisms are available. Nevertheless, Chlorhexidine oral decontamination seems promising. Further studies are clearly needed in testing the effect of Chlorhexidine in specific populations with standard protocols (which includes specific concentration, frequency, and type of agents) to generalize the findings. Studies also may be done to test the effect of different oral antiseptics in reducing VAP, so as to enrich the body of knowledge within this area.
|
Acknowledgements The author is grateful to B.B Dixit Library, AIIMS, New Delhi India, for their guidance in retrieving online journals for this meta-analysis. Competing Interests None declared Author Details E Balamurugan R.N, R.M, M.Sc., Lecturer, Vinayaka Missions College of Nursing, Vinayaka Missions University, Pudhucherry, India. A Kanimozhi R.N, R.M, M.Sc, Associate Professor , Vinayaka Missions College of Nursing, Vinayaka Missions University, Pudhucherry, India. Govinda Kumari R.N, R.M, M.Sc., Lecturer, Vinayaka Missions College of Nursing, Vinayaka Missions University, Pudhucherry, India. CORRESPONDENCE: E Balamurugan R.N, R.M, M.Sc., Lecturer, Vinayaka Missions College of Nursing, Vinayaka Missions University, Pudhucherry, India. Email: bmbalanursing@gmail.com |
References
1.Vincent JL, Bihari DJ, Suter PM. The prevalence of nosocomial infection in intensive care units in Europe: results of the European Prevalence of infection in Intensive Care (EPIC) Study: EPIC International Advisory Committee. JAMA 1995; 274:639-644.
2.Richards MJ, Edwards JR, Culver DH, Gaynes RP. Nosocomial infections in medical intensive care units in the United States. National Nosocomial Infections Surveillance System. Crit Care Med 1999; 27:887-892.
3.Centers for Disease Control and Prevention. National Nosocomial Infections Surveillance (NNIS) System report, data summary fromJanuary 1992 through June 2004, issued October 2004. Am J Infect Control 2004; 32:470-485.
4.Safdar N, Dezfulian C, Collard HR, Saint S. Clinical and economic consequences of ventilator-associated pneumonia: a systematic review. Crit Care Med 2005; 33:2184-2193.
5.Danchaivijitr S, Dhiraputra C, Santiprasitkul S, Judaeng T. Prevalence and impacts of nosocomial infection in Thailand 2001. J Med Assoc Thai 2005; 88(suppl 10):S1-S9.
6.Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med 2002;165:867-903.
7.Rello J, Ollendorf DA, Oster G, Vera-Llonch M, Bellm L, Redman R. Epidemiology and outcomes of ventilator-associated pneumonia in a large US database. Chest 2002;122:2115-21.
8.Tablan OC, Anderson LJ, Besser R, Bridges C, Hajjeh R. Guidelines for preventing health-care-associated pneumonia, 2003: recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee. MMWR Recomm Rep 2004;53:1-36.
9.American Thoracic Society, Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005;171:388-416.
10.Estes RJ, Meduri GU. The pathogenesis of ventilator-associated pneumonia: Mechanisms of bacterial transcolonisation and airway inoculation. Intensive Care Med 1995;21:365-83.
11.Bagg J, MacFarlane TW, Poxton IR, Miller CH, Smith AJ. Essentials of Microbiology for Dental Students. 3rd ed.New York: Oxford University Press, 1999:227-310.
12.Gibbons RJ. Bacterial adhesion to oral tissues: a model for infectious diseases. J Dent Res 1989;68(5):750-760.
13.Angela M. Berry, Patricia M. Davidson, Janet Masters and Kaye Rolls. Systematic Literature Review of Oral Hygiene Practices for Intensive care Patients Receiving Mechanical Ventilation. Am J Crit Care 2007;16:552-562
14.Ee Yuee Chan, Annie Ruest, Maureen O Meade, Deborah J Cook. Oral decontamination for prevention of pneumonia in mechanically ventilated adults: systematic review and
meta-analysis. BMJ2007;334:861.
15.Selective Decontamination of the Digestive Tract Trialists’ Collaborative Group. Meta-analysis of randomised controlled trials of selective decontamination of the digestive tract. BMJ 1993;307:525-32.
16.Brun-Buisson C, Legrand P, Rauss A, Richard C, Montravers F, Besbes M. Intestinal decontamination for control of nosocomial multiresistant gram-negative bacilli. Study of an outbreak in an intensive care unit. Ann Intern Med 1989;110:873-81
17.Pittet D. Improving compliance with hand hygiene. In: Wenzel RP, ed. Prevention and control of nosocomial infections, 4th ed. Philadelphia: Lippincott Williams, and Wilkins.;2003.p.532-3.
18.Pineda LA, Saliba RG, El Solh AA. Effect of oral decontamination with chlorhexidine on the incidence of nosocomial pneumonia: a metaanalysis. Crit Care 2006;10:R35.
19.Koeman M, van der Ven AJ, Hak E. Oral decontamination with chlorhexidine reduces the incidence of ventilatorassociated pneumonia. Am J Respir Crit Care Med 2006;
173(12):1348-1355.
20.Rello J, Koulenti D, Blot S. Oral care practices in intensive care units: a survey of 59 European ICUs. Intensive Care Med. 2007 Jun;33(6):1066-70
21.DeRiso AJ II, Ladowski JS, Dillon TA, Justice JW, Peterson AC. Chlorhexidine gluconate 0.12% oral rinse reduces the incidence of total nosocomial respiratory infection and
nonprophylactic systemic antibiotic use in patients undergoing heart surgery. Chest. 1996;109(6):1556-1561.
22.Fourrier F, Cau-Pottier E, Boutigny H, Roussel-Delvallez M, Jourdain M, Chopin C. Effects of dental plaque antiseptic decontamination on bacterial colonization and nosocomial
infections in critically ill patients. Intensive Care Med. 2000;26(9):1239-1247
23.Houston S, Hougland P, Anderson JJ, LaRoccoM, Kennedy V, Gentry LO. Effectiveness of 0.12% chlorhexidine gluconate oral rinse in reducing prevalence of nosocomial pneumonia in patients undergoing heart surgery. Am J Crit Care 2002;11:567-70.
24.MacNaughton P, Bailey J, Donlin N. , Intensive Care Med, A randomized controlled trial assessing efficacy of oral chlorhexidine in ventilated patients: European Society of Intensive Care Medicine2004;30( suppl): S5–S18.
25.Fourrier F, Dubois D, Pronnier P. Effect of gingival and dental plaque antiseptic decontamination on nosocomial infections acquired in the intensive care unit: a double-blind
placebo-controlled multicenter study. Crit Care Med 2005; 33(8):1728-1735.
26.Segers P, Speekenbrink RG, Ubbink DT. Prevention of nosocomial infection in cardiac surgery by decontamination of nasopharynx and oropharynx with chlorhexidine gluconate: a randomized controlled trial.JAMA 2005; 296:2460–2466.
27.Bopp M, Darby M, Loftin KC, Broscious S.Effects of daily oral care with 0.12% chlorhexidine gluconate and a standard oral care protocol on the development of nosocomial pneumonia in intubated patients: a pilot study. J Dent Hyg 2006;80(3):9.
28.Hutsaya Tantipong, Chantana Morkchareonpong, Songyod Jaiyindee, Visanu Thamlikitkul. Randomized Controlled Trial and Meta-analysis of Oral Decontamination with 2% Chlorhexidine Solution for the Prevention of Ventilator-Associated Pneumonia. Infection control and hospital epidemiology 2008;29(1):345-350
29.Johanson WG Jr, Pierce AK, Sanford JP. Changing pharyngeal bacterial flora of hospitalized patients: emergence of gram-negative bacilli. N Engl J Med 1969;281:1137–1140.
30.Johanson WG Jr, Pierce AK, Sanford JP, Thomas GD. Nosocomial respiratory infections with Gram-negative bacilli: the significance of colonization of the respiratory tract. Ann Intern Med 1972;77:701–706.
31.Bonten MJM, Bergmans DCJJ, Ambergen AW, de Leeuw PW, van der Geest S, Stobberingh EE, Gaillard CA. Risk factors for pneumonia, and colonization of respiratory tract and stomach in mechanically ventilated ICU patients. Am J Respir Crit Care Med 1996;154:1339– 1346.
32.Garrouste-Org M, Chevret S, Arlet G, Marie O, Rouveau M, Popoff N, Schlemmer B. Oropharyngeal or gastric colonization and nosocomial pneumonia in adult intensive care unit patients: a prospective study based on genomic DNA analysis. Am J Respir Crit Care Med 1997; 156:1647–1655
33.Viola´n JS, Ferna´ndez JA, Bordes-Benı´tez A, Cardenosa-Cendrero JA, de Castro FR. Impact of quantitative invasive diagnostic techniques in the management and outcome of mechancally ventilated patients with suspected pneumonia. Crit Care Med 2000;28:2737–2741.
34.Russell AD, Day MJ. Antibacterial activity of chlorhexidine. J Hosp Infect 1993;25:229–238.
35.Stoutenbeek CP, van Saene HK, Miranda DR, Zandstra DF. The effect of selective decontamination of the digestive tract on colonisation and infection rate in multiple trauma patients. Intensive CareMed 1984;10:185-92.
36.Vandenbroucke-Grauls CM, Vandenbroucke JP. Effect of selective decontamination of the digestive tract on respiratory tract infections and mortality in the intensive care unit. Lancet 1991;338:859-62.
37.Selective Decontamination of the Digestive Tract Trialists’ Collaborative Group. Meta-analysis of randomised controlled trials of selective decontamination of the digestive tract. BMJ 1993;307:525-32
38.LiberatiA,D’Amico R, Pifferi, Torri V,Brazzi L. Antibiotic prophylaxis to reduce respiratory tract infections and mortality in adults receiving intensive care. Cochrane Database Syst Rev 2004;(1):CD000022
39.Chlebicki MP, Safdar N. Topical chlorhexidine for prevention of ventilator-associated pneumonia: a meta-analysis. Crit Care Med 2007;35:595-602.
40.Zamora Zamora F.(Effectiveness of oral care in the prevention of ventilator-associated pneumonia. systematic review and meta-analysis of randomised clinical trials.. Enferm Clin. 2011 Nov-Dec;21(6):308-19

The above article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.




