Do thalidomides have a role in the treatment of multiple sclerosis?
G.V. Sherbet
Cite this article as: BJMP 2015;8(3):a828
|
|
Abstract Angiogenesis is pivotal component of many normal biological programmes as well as of pathogenetic processes involved in tumour growth and progression and of inflammatory and autoimmune diseases such as multiple sclerosis (MS), a demyelinating disease of the CNS. Many angiogenic factors are expressed in MS and in the animal model of MS known as experimental autoimmune encephalomyelitis. Inhibition of angiogenesis by suppressing these angiogenic effectors or inhibiting the elements of angiogenic signalling might provide a viable way to target therapy to manage MS. The focus of this article is on the ability of thalidomide and its analogues to inhibit angiogenic signalling systems. Thalidomide is a highly toxic drug but its analogues, lenalidomide and pomalidomide, show reduced toxicity and greater efficacy of growth suppression and inhibition of angiogenesis. The thalidomides are highly efficient suppressors of canonical and non-canonical angiogenic signalling by PI3K (phosphoinositide-3 kinase)/Akt, NF (nuclear factor)-κB and mTOR (mammalian target of rapamycin). Here a postulate is presented that the perceived potential synergy between the thalidomides and modulators of angiogenic signalling might deliver benefits of thalidomides more effectively and at lower dosages compatible with greater safety of administration. Keywords: Multiple sclerosis; angiogenesis signalling; thalidomides |
Angiogenesis is an integral process in biological programmes of embryonic development, tissue damage and regeneration, tumour growth and progression and pathogenesis of inflammatory and autoimmune diseases. MS (multiple sclerosis) is a demyelinating disease of the CNS (central nervous system). Angiogenesis has been a consistent feature of demyelinating plaques of MS1-3. Many inducers of angiogenesis are expressed in these plaques. They are also closely associated with the animal model of MS viz. EAE (experimental autoimmune encephalomyelitis)4 (Table 1). This has led to the suggestion that inhibition of angiogenesis by suppressing these effectors or inhibiting the elements of angiogenic signalling pathways might provide a viable way to target therapy to manage MS.
Table 1. Angiogenic mediators of MS
| Angiogenic agent/mediator |
| Vascular endothelial growth factor (VEGF)/VEGF receptor 2 (VEGFR2) Nitric oxide (NO) and NOS (NO synthase) Transforming growth factor-β (TGF-β) Basic fibroblast growth factor (bFGF) ↓ Matrix metalloproteinases (MMP) Hepatocyte growth factor (HGF) |
[Note: Inhibitory effects of thalidomides were described by Sherbet4; D’Amato et al.6; Kenyon et al.7; Lu et al.8]
Multiple sclerosis is an autoimmune inflammatory condition and so immunomodulators have been used in treatment. It is recognised that aberrant activation of the immune system and the associated network of its regulation are important events in the pathogenesis of the disease. This is the rationale for using immunomodulatory agents in disease control. Among immunomodulators of note are Fingolimod which prevents infiltration of auto-destructive lymphocytes into the CSF, Teriflunomide which reduces lymphocyte infiltration of the CNS, axonal loss and inflammatory demyelination, and dimethyl fumarate, which modulates the immune system by many mechanisms. Furthermore, much attention has been devoted to the immunomodulatory properties of MSCs (mesenchymal stem cells) 4,5. Thalidomides are also capable of modulating the function of key element of the immune system related to the pathogenesis of MS, but this brief article is intended to emphasise the potential of thalidomide and its analogues as potent inhibitors of angiogenesis and the latent possibility of their use as a therapeutic agent in the control of MS.
Thalidomide was introduced over four decades ago to treat respiratory infections and to combat morning sickness in pregnant women. It was withdrawn when it was found to be highly teratogenic. The teratogenic effects are a result of the binding of thalidomide to cereblon, a protein found in both embryonic and adult tissues. Cereblon is required for normal morphogenesis. It is inactivated by binding to thalidomide and this leads to teratogenesis9. Thalidomide possesses immunomodulatory, anti-inflammatory, anti-angiogenesis and cell proliferation inhibitory properties and this has suggested its use in the treatment of cancer5. Analogues of thalidomide, viz. lenalidomide and pomalidomide, have been synthesised and these possess reduced toxicity and greater efficacy10, 11. Recently, many studies have elucidated the signalling pathways which thalidomides inhibit and thereby suppress cell proliferation, promote apoptosis and inhibit angiogenesis. These have led to the suggestion of combining the modulators of these signalling pathways to synergise with thalidomides to deliver the suppressor effects with enhanced efficacy and at lower concentrations thus reducing the side effects5 (Figure 1).
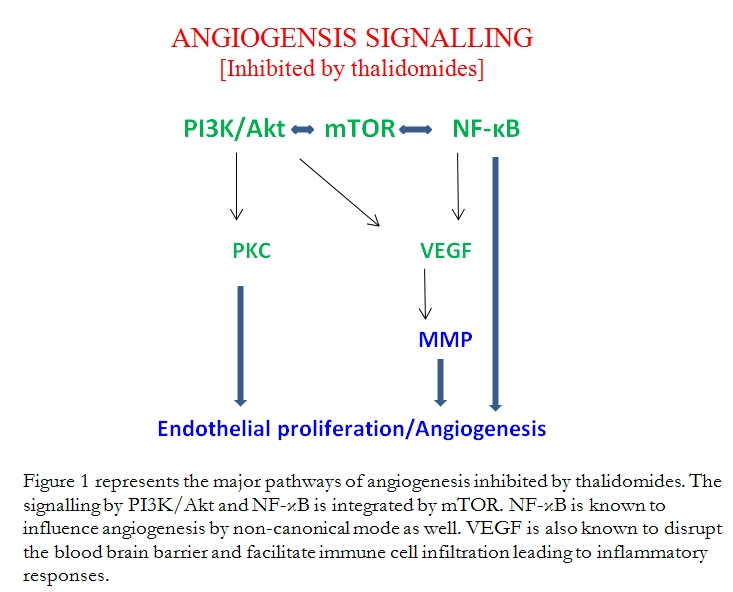
Most of the work on the efficacy of thalidomide and the analogues has been carried out in preclinical models. Quite understandably, in the clinical setting very little effort is seen to check whether thalidomide or the analogues provide any beneficial effects in MS or neuro-inflammation. Clinically orientated investigations so far relate mainly to multiple myeloma and some other forms of haematological malignancies but not solid tumours5. Any perceived beneficial effects are probably outweighed by the side effects. We need to expend more effort and design and develop new analogues with reduced toxicity. In this context one should emphasise that pre-clinical exploration of the potential synergy between the thalidomides and the acknowledged modulators of the signalling pathways would be worthwhile. This might enable the delivery of benefits more effectively and at lower dosages. It is needless to say that safety of drug administration is of paramount importance.
|
Competing Interests None declared Author Details G.V. SHERBET, DSc, FRSC, FRCPath, Institute for Molecular Medicine, Huntington Beach CA, USA and University of Newcastle upon Tyne UK. CORRESPONDENCE: G.V. SHERBET, Institute for Molecular Medicine, Huntington Beach CA, USA and University of Newcastle upon Tyne UK. Email: gsherbet@immed.org |
References
- Holley, JE., Newcombe, J., Whatmore, JL, Gutowski NJ. Increased blood vessel density and endothelial cell proliferation in multiple sclerosis cerebral white matter. Neurosci Lett 2010; 47: 65-70.
- Lengfeld, J., Cutforth, T., Agalliu, D. The role of angiogenesis in the pathology of multiple sclerosis. Vasc cell 2014; 6: 23-9.
- Girolamo, F., Coppola, C., Ribatti, D., Trojano M. Angiogenesis in multiple sclerosis and experimental autoimmune encephalomyelitis. Acta Neuropathol Commun 2014; 2: 84.
- Sherbet, GV. Molecular approach to targeted therapy for multiple sclerosis (submitted). (2015).
- Sherbet, GV. Therapeutic potential of thalidomide and its analogues in the treatment of cancer. Anticancer Res 2015; in press.
- D’Amato, RJ., Loughnan, MS., Flynn, E., Folkman, J. Thalidomide is an inhibitor of angiogenesis, Proc. Natl. Acad. Sci. USA 1994: 91: 4082–4085.
- Kenyon, BM., Browne, F., D’Amato, RJ. Effects of thalidomide and related metabolites in a mouse corneal model of neovascularization, Exp Eye Res 1997; 64: 971–978.
- Lu, L., Payvandi, F., Wu, L., Zhang, LH., Hariri, RJ., Man HW. et al. The anti-cancer drug lenalidomide inhibits angiogenesis and metastasis via multiple inhibitory effects on endothelial cell function in normoxic and hypoxic conditions. Microvasc Res 2009; 77: 78-86.
- Ito, T., Ando, H., Suzuki, T., Ogura, T., Hotta, K., Imamura, Y. et al, Identification of a primary target of thalidomide teratogenicity, Science 2010; 327: 1345-1350.
- Botting, J. The history of thalidomide, Drug News Perspect. 2002; 15: 604-611.
- Bartlett, JB., Dredge, K., Dalgleish, AG. The evolution of thalidomide and its IMiD derivatives as anticancer agents, Nature Rev Cancer 2004; 4: 314-322.

The above article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.




