Denosumab-associated Osteonecrosis of the Jaw; A Case Series and Literature Review
Elena Kyriakidou,Mohamed Badr, Simon Atkins and Sheelah Harrison
Cite this article as: BJMP 2016;9(4):a930
|
|
Abstract Introduction: Medication-related osteonecrosis of the jaw (MRONJ) is a severely debilitating condition of multifactorial pathogenesis. It primarily involves patients receiving intravenous bisphosphonates (BPs) and most recently the new antiresorptive drug, denosumab, for the treatment of skeletal-related malignancies. There is no curative treatment and no consensus exists regarding the clinical management of such patients. This review aims to share our current clinical experience at Sheffield Teaching Hospitals’ Trust and raise awareness of the increase in severity of ONJ in patients receiving denosumab. Abbreviations: MRONJ- Medication-related osteonecrosis of the jaw; BPs- Bisphosphonates; SREs- skeletal-related events; IV- intravenous; RANKL-Receptor activator of nuclear factor kappa-B ligand; MM- Multiple myeloma; ONJ- Osteonecrosis. |
Introduction
Metastatic bone disease is a relatively common event in the advanced stages of many malignancies.1 Bone-modifying agents decrease the incidence of skeletal-related events (SREs) such as spinal cord compression and bone fracture, as well as the need for skeletal radiotherapy or surgery.2
Bone modifying agents such as intravenous bisphosphonates (IV BPs) (e.g. pamidronate and zoledronic acid) and denosumab are approved for prevention of SREs. IV BPs are primarily used and effective in the treatment and management of cancer related conditions such as multiple myeloma (MM), and breast cancer with skeletal metastases, because they reduce bone pain, hypercalcemia, and the risk of pathologic fractures.3
Denosumab, a receptor activator of nuclear factor kappa-B ligand (RANKL) inhibitor, represents a breakthrough in the treatment of osteoporosis, MM, and bone metastases. The Food and Drug Administration (FDA) approved it in 2010 for the prevention of SREs in patients with bone metastases and in 2011 for the prevention of endocrine-therapy induced bone loss in patients taking aromatase inhibitors for breast cancer and in patients with non-metastatic prostate cancer.
Three international, randomised, double-blind, double-dummy phase III studies have evaluated denosumab versus zoledronic acid for the treatment of SREs in breast and prostate cancers, and in combined solid tumours and MM. Denosumab’s superior efficacy over zoledronic acid was demonstrated in the studies of patients with advanced breast or prostate cancer, as well as in a pre-specified integrated analysis of all patients enrolled across the three studies.4
In the 2014 position paper of the American Association of Oral and Maxillofacial Surgeons (AAOMS), the nomenclature “bisphosphonate-related osteonecrosis of the jaw” changed to “medication related osteonecrosis of the jaw” (MRONJ). MRONJ is defined as cases in which all of the following 3 characteristics are present5:
- current or previous treatment with antiresorptive or antiangiogenic agents
- exposed bone or bone that can be probed through an intraoral or extra-oral fistula in the maxillofacial region that has persisted for longer than 8 weeks
- no history of radiation therapy to the jaws or obvious metastatic disease to the jaws
Other terminologies used previously include “denosumab related osteonecrosis of the jaw” (DRONJ), and “antiresorptive agent-induced ONJ” (ARONJ).
The aetiopathogenesis of MRONJ related to denosumab therapy remains enigmatic, and hypotheses have focused on reduced bony turnover, infection, toxicity of the soft tissue, and antiangiogenesis. The epidemiology also remains unclear, and reported incidence varies widely.6 Overall, it is estimated that bone necrosis can develop in about 0.7-1.9% of patients with malignancy who are given high-potency IV BPs (such as zoledronic acid), and in 0.01–0.1% of those with osteoporosis who take low-potency oral BPs (such as alendronate). Data relevant to denosumab given subcutaneously in patients with metastatic cancer and osteoporosis seem to replicate those when IV high-potency BPs are administered.7 The risk of osteonecrosis of the jaw (ONJ) is higher in patients exposed to concomitant antiagiogenic medication. The individuals’ risk of ONJ is further determined by factors such as the potency of agent, cumulative dosage or duration of antiresorptive treatment, route of administration, comorbidities and local factors such as periodontal disease.8,9 Oral hygiene plays a significant role with evidence supporting a strong correlation between bacteria associated with periodontal disease and MRONJ.10
MRONJ typically manifests as painful and often infected areas of necrotic bone, which subsequently may lead to severe chronic pain and facial disfigurement. This adversely affects the ability to eat, speak and lowers the quality of life. Adverse events related to RANKL inhibitors are usually considered to be infrequent and low in occurrence. Unfortunately from our recent clinical experience at Sheffield Teaching Hospitals' Trust, there have been several new cases presented in a very short period of time. In this paper we present a case series of MRONJ related to denosumab therapy since adverse events of denosumab in the mandible or maxilla have received relatively little attention.
The aim of this article is to highlight the elevated risk of MRONJ in patients receiving denosumab treatment and educate all health care providers involved in the management of such patients. Furthermore, the mechanisms of denosumab, comparison with bisphosphonates and the reported management strategies are reviewed.
Mechanism of Denosumab
Denosumab is an antiresorptive agent that exists as a human IgG2 monoclonal antibody and inhibits the binding of the receptor activator of nuclear factor kappa-B ligand (RANKL) to RANK (Receptor Activator of Nuclear Factor kappa-B). The binding normally signals the proliferation of osteoclasts, as RANK is expressed on the surface of osteoclasts and their precursors, whereas its ligand, RANKL, is a membrane bound protein expressed by bone marrow stromal cells, osteoblasts and T-lymphocytes. The activation of RANK is integral to the function of osteoclasts. Osteoprotegerin binds to membrane bound RANKL on osteoblast which in turns decreases the osteoclastic activity and in theory negatively effects bone turnover. Denosumab acts similarly to osteoprotegerin but has a higher affinity for RANKL.11-13
Denosumab follows nonlinear, dose-dependent pharmacokinetics. The bioavailability of one subcutaneous denosumab injection is 61% and serum concentrations are detected within 1 hour. Maximum serum concentrations occur in 5-21 days and cessation of osteoclast activity occurs within six hours of the subcutaneous injection. The normal function is restored approximately six to nine months later, whilst bone turnover returns to normal shortly after this.14 Based upon monoclonal antibody pharmacokinetics, denosumab is most likely cleared by the reticuloendothelial system with minimal renal filtration and excretion thus avoiding nephrotoxicity. Its elimination half-life is 32 days, and it does not incorporate into bone.15
It is currently marketed as Prolia® and Xgeva®, approved by FDA. Prolia® is administered subcutaneously every six months and has shown to reduce the incidence of new vertebral, non-vertebral, and hip fractures in osteoporotic patients.16,17 Xgeva® is also effective in reducing SRE related to metastatic bone disease from solid tumours when administered intravenously on a monthly basis.17,18
RANKL Inhibitors and BPs Pharmacokinetics
There are fundamental differences between denosumab and BPs with regard to their mode of action. Denosumab is an antibody and acts extracellularly whereas BPs act intracellularly. As such, BPs must be present in the circulation and available for reuptake into bone for prolonged periods to function.19 There is not any evidence of drug recycling with RANKL inhibitors, and therefore it is suggested that their adverse effects can be reversible with discontinuation, in fact leading to a transient rebound phenomenon, which can be restored, with subsequent treatment.14,20 On the other hand, recycling of BPs in the circulation system has been proposed as a reason for the long duration of action even after cessation which can be up to 12 years.
The US FDA-approved manufacturer’s package insert for both zolendronate and pamidronate states that “there are no data available to suggest whether discontinuation of bisphosphonate treatment reduces the risk of ONJ in patients who require dental procedures during therapy and that clinical judgment of the treating physician should guide the management plan of each patient based on individual benefit/ risk assessment”. The package insert for denosumab does not address the issue of treatment continuation in patients who develop MRONJ to date.
Denosumab is a circulating protein capable of distributing throughout extravascular space. It is expected to reach all sites within bone including intracortical sites unlike with BPs. BPs have strong affinity for hydroxyapatite and bone mineral which limits their even distribution throughout the skeleton, particularly to sites deep within the bone.19,21 This can explain the more profound inhibition of bone remodelling with denosumab than that seen with BPs.
Case Series
Case 1
A 55 year-old lady referred to a dedicated Oral Surgery nerve injury clinic for an opinion and management of her left sided inferior alveolar nerve (IAN) paraesthesia. The patient presented with a history of numbness in the left sided inferior alveolar nerve distribution following removal of the left mandibular second premolar (LL5) in July 2014. She was asymptomatic until she had the LL5 removed and since had suffered with constant pain and numbness. A year later, she had removal of the left mandibular first molar (LL6) and gave a history of recurrent infections and excruciating pain in her mandible over the past two months. On presentation she had an obvious submental swelling and left sided IAN anaesthesia.
Medically she was diagnosed with breast cancer in 2011, for which she underwent wide local excision followed by chemotherapy. She then was placed on unknown clinical trial that we identified at the time to be denosumab trial, following liaison with the Oncology team. She is currently receiving intravenous denosumab every three months.
Clinical examination revealed a grossly mobile anterior mandible with widespread bony necrosis and associated osteomyelitis. Sensory testing revealed complete anaesthesia in the left sided IAN distribution secondary to MRONJ.
An OPG (Orthopantogram) and CBCT (Cone-Beam Computerised Tomography) revealed an extensive patchy area of ill-defined bone loss in the anterior mandible extending posteriorly to the premolar/molar areas bilaterally (Fig 1).
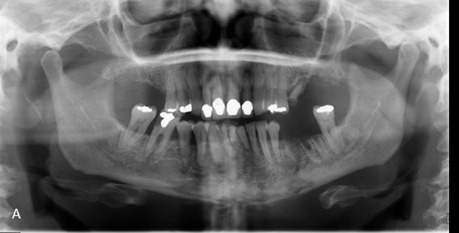
Figure 1 A) OPG showing non-healing sockets in the left mandible with extensive bony destruction together with periosteal reaction extending to the right mandible as shown by the arrows.
Rather interestingly, the bony destruction was evident bilaterally with the patient only having had extraction of teeth in the left mandible (Fig 1). This could be the case of spontaneous ONJ in the right mandible or an extensive ONJ arising from simple extractions on the left side.
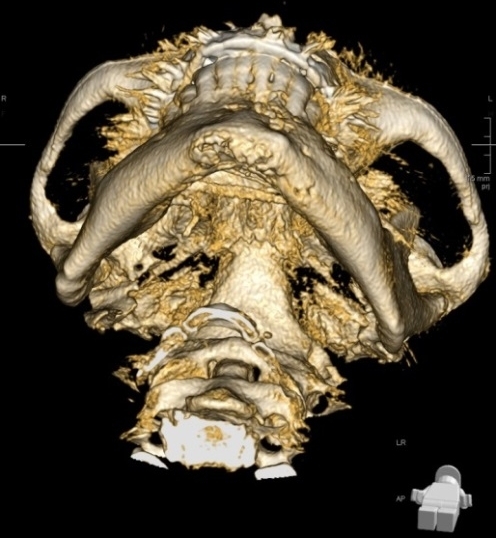
Figure 2 3D reconstruction of the CBCT image demonstrating extensive bony destruction involving the lower border of anterior mandible in keeping with a spreading chronic bony infection and clinical presentation of submental swelling as showing by arrows.
Case 2
A 66-year-old female referred by her general medical practitioner (GMP) with a 3-month history of delayed healing following a tooth extraction in the left posterior mandible. She had moderate to severe discomfort and reported multiple previous infections and purulent discharge from the area, which treated with multiple courses of antibiotics. In addition, she reported discomfort from the root treated right mandibular first and second premolar teeth (LR4 and LR5).
Medically she was diagnosed with breast cancer over 10 years ago for which she underwent resection followed by chemotherapy. Three years ago, she diagnosed with metastatic deposits and therefore has been receiving intravenous denosumab every six weeks since then. Other medications include steroids, chemotherapy agents, antihypertensives and analgesics. She did not receive any radiotherapy or BPs treatment in the past.
Clinical presentation revealed a heavily restored dentition with chronic generalised periodontal disease. There was evidence of widespread bone loss clinically and radiographically. The slow healing socket in the left mandible was visible but did not have any exposed bone (Fig 3). The lower right first and second premolar teeth (LR4 and LR5) were clinically and radiographically sound.
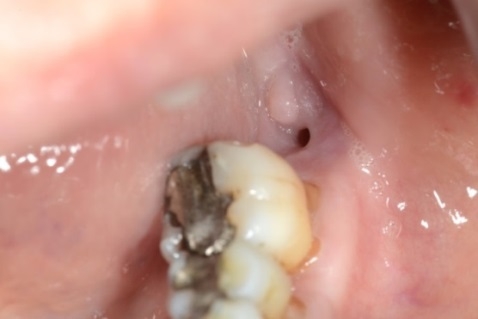
Figure 3. Non-healing socket in the left posterior mandible with no evidence of exposed bone or suppuration as showing by white arrow. Gingiva recession (black arrows) is evident in the LL6 and LL5 teeth in keeping with chronic periodontal disease.

Figure 4 Coronal sections of CBCT A and B showing multiple lytic areas within the inferior cortex of the mandible and incomplete healing of the extraction sockets.
On follow-up appointments, the patient suffered multiple repeated infections in the right and left posterior mandible and due to deteriorating periodontal disease, the LR4, LR5, LR6 were extracted by her own general dental practitioner (GDP) due to severe mobility. All three extraction sockets failed to heal (Fig 5) leading to an extensive area of exposed bone in the right mandible, extending from the lower right first premolar (LR4) to lower left first molar (LL6) region. Conservative management was embarked which included antibiotics, chlorhexidine mouthwash and routine oral hygiene appointments. Selective sharp bone trimming and three sequestrectomies were undertaken. At the same time, liaison with the patient’s oncologist resulted in cessation of the denosumab therapy and complete resolution of her oral symptoms.
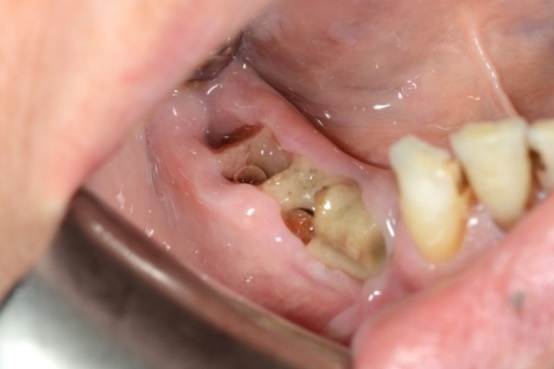
Figure 5 Clinical picture of exposed necrotic bone (white arrows) following simple extractions of periodontally involved teeth.
Case 3
A 76-year-old lady referred to the Oral Surgery department by her GDP with a 3-month history of a non-healing lower left first premolar (LL4) socket. The patient was treated with two courses of antibiotics prior to referral which provided only temporary relief to her symptoms.
Medically she was diagnosed with breast cancer 10 years ago and recently commenced intravenous denosumab for metastatic disease. She also receives hormone therapy and palliative radiotherapy to the spine.
On clinical examination, there was a partially healed LL4 socket with a rather granulomatous appearance. There was no clinical evidence of suppuration or bony exposure. Radiographs confirmed the absence of bony infill in the socket. Local debridement and biopsy of the granulomatous tissue was performed to exclude any metastatic disease. Biopsy report confirmed the presence of inflammation tissue.
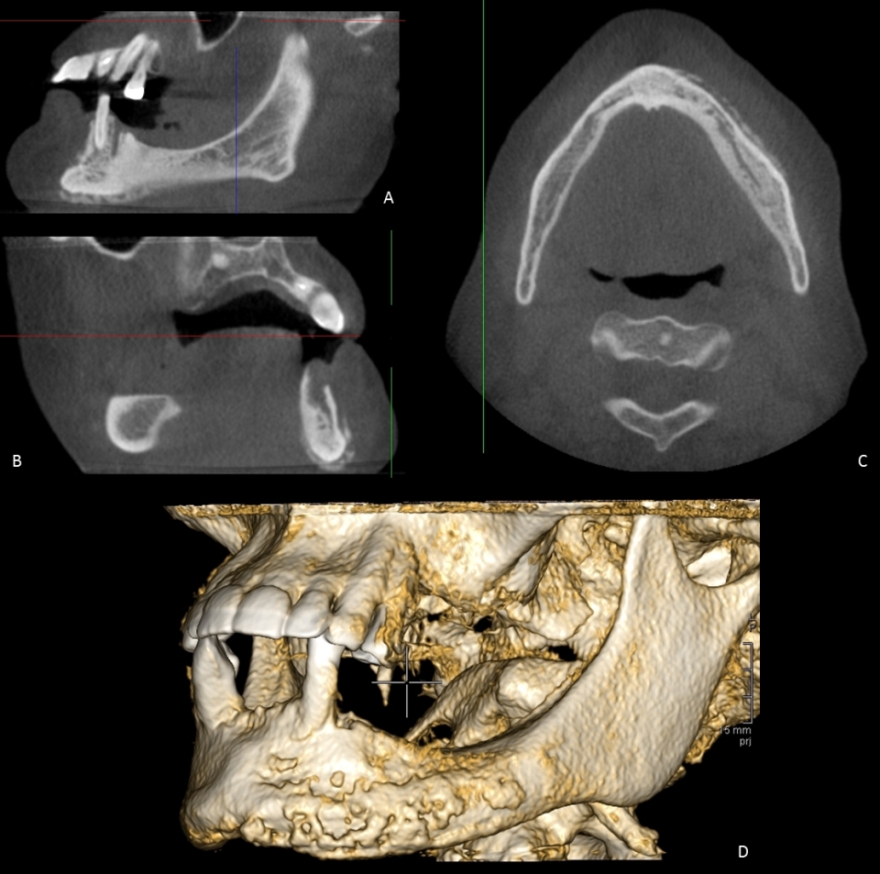
Figure 6 CBCT scan; A and B sagittal views, C axial view and D 3D reconstruction. Extensive periosteal reaction extending from the midline of the mandible to the left molar region is evident in keeping with chronic osteomyelitis secondary to MRONJ.
Liaison with the microbiologist suggested a long-term antibiotic course to arrest osteomyelitis. Further liaison with the oncology team, resulted in denosumab being stopped for 4 months. On subsequent review appointments, patient’s symptoms improved however, there is now an area of exposed bone in the LL4 region as shown in Fig 7.
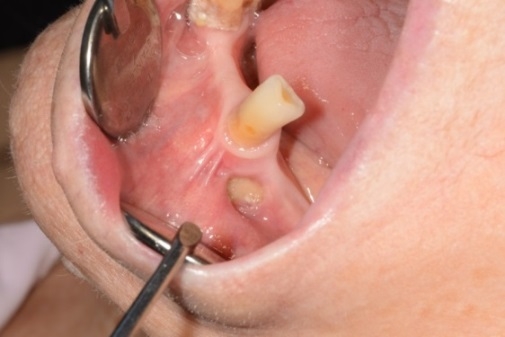
Figure 7 Clinical photo illustrating exposed bone (white arrow) in the LL4 region without evidence of local infection.
Case 4
A 65-year-old lady referred to the Oral Surgery department by her GDP with a history of a sore upper mouth and jaw underneath the dentures which is unable to wear.
Medically she was diagnosed with disseminated breast malignancy including bone metastases 3 years ago and for that, she is on exemestane and IV Denosumab monthly.
Clinical examination revealed multiple draining sinuses in the anterior maxilla. There was a partially healed LL4 socket with a rather granulomatous appearance and tenderness on palpation. There was neither discharge from the area nor any exposed bone. Radiographs confirmed the absence of bony infill in the LL4 socket. Local debridement and biopsy of the granulomatous tissue was performed to exclude any potential malignancy and it was confirmed as inflammation tissue.
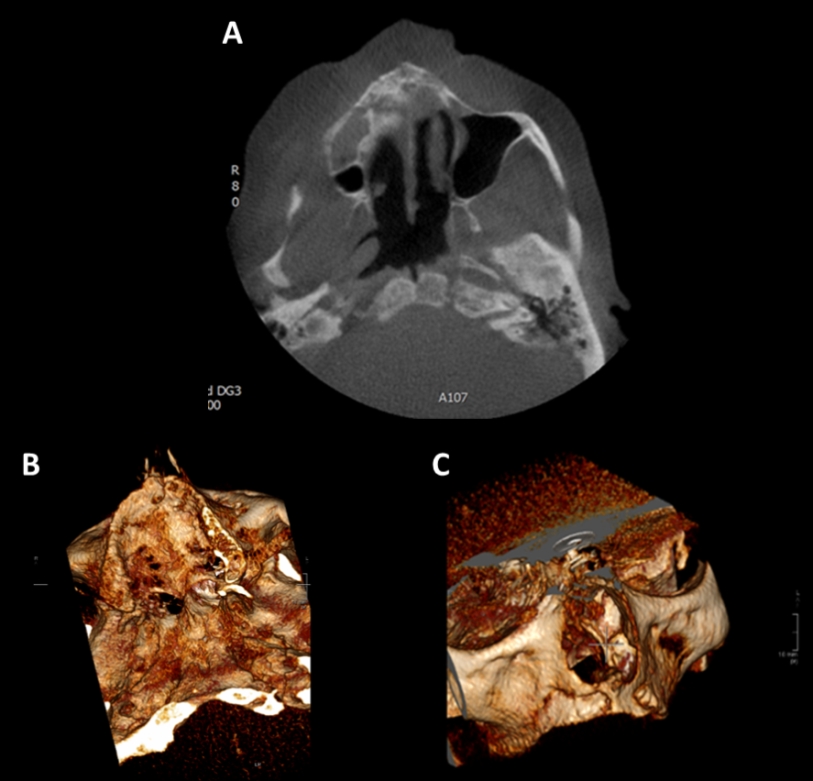
Figure 8 CBCT scan; A axial view, B and C 3D reconstruction. A 25mm fragment of right anterior maxilla is beginning to sequestrate. This extends from the anterior margin of the right maxillary sinus approximately to the position of the upper left lateral incisor, crossing the midline. The sequestrated fragment involves the lateral margin of the nasal cavity. There is bilateral moderate mucosal thickening in the maxillary sinuses. Extensive periosteal reaction extending from the midline of the mandible to the left molar region is evident in keeping with chronic osteomyelitis secondary to MRONJ.
Table 1 Summary of cases
| Cases | Indications | Duration (months) | Clinical Findings |
| Case 1 | Metastatic deposits from primary breast malignancy | 48 |
Anaesthesia in the distribution of the left inferior alveolar nerve |
| Case 2 | Metastatic deposits from primary breast malignancy | 36 | Chronic generalised adult periodontal disease Non-healing extraction sockets Exposed bone persisted for longer than 8 weeks Severe pain |
| Case 3 | Metastatic deposits from primary breast malignancy and myeloma | 24 | Non-healing extraction socket with granulomatous tissue Severe pain |
| Case 4 | Disseminated breast malignancy including bone metastases | 30 | Multiple draining sinuses in anterior maxilla Non-healing extraction socket with granulomatous tissue Severe pain |
Discussion
ONJ associated with antiresorptive therapy deserves distinction from other causes and diseases/medications associated with the development of osteonecrosis of the jaw. AAOMS recently published stage specific treatment recommendation for MORNJ.22 The various stages and suggested stage-specific treatment strategies are not evidence-based, and in particular, stage 0 disease is not universally accepted. AAOMS recommendations echoed those stated in previous years for BRONJ, namely supporting conservative therapy, with aggressive surgery offered only to symptomatic patients. In contrast, the MRONJ guideline report from the German Dental and the German Oral and Maxillofacial Associations refrains from recommending therapy at least for certain stages of the disease. This might be attributed to the pitfalls of current MRONJ criteria. Furthermore, due to poor guidelines specifically related to RANKL inhibitors, no agreement exists on a universally acceptable therapy strategy of such cases.
Management strategies are largely based on expert opinion rather than experimental data. It includes prevention, conservative and surgical modalities. Prevention of the condition is the gold standard. It is highly recommended all patients have a comprehensive dental examination and preventive dentistry (pre-emptive extraction of unsalvageable teeth and optimised periodontal health) before commencing antiresorptive therapy.23,24 Oral hygiene should be kept meticulous during the course of therapy as periodontal disease and associated bacteria claim to be implicated in this condition and also observed in these cases.
The success rate of conservative treatment regimens range from less than 20% 25,26 to above 50%27,28 although some cases become chronic and develop complications.29
Microbial cultures from areas of exposed bone are not always helpful since normal oral microbes are isolated. However, when there is extensive soft tissue involvement, microbial cultures may help to define comorbid oral infections, which may guide the selection of an appropriate antibiotic regimen.30
Regardless of the stage of disease, areas of necrotic bone that are a source of chronic soft tissue irritation and loose bony sequestra should be removed or recontoured so that soft tissue healing can be optimised. This is in line with our clinical experience. The extraction of symptomatic teeth within exposed, necrotic bone should be considered as it appears unlikely that extraction will worsen the established necrotic process. Otherwise, surgical resection of necrotic bone should generally be reserved for refractory or advanced cases.31 Resection may occasionally result in even larger areas of exposed and painful infected bone.32
A recently published MISSION study7 reported that the AAOMS system misclassified/ underestimated the severity of the disease at a rate of about 1 in 3, in particular in patients suffering from MRONJ stage 1 and 2. The authors conclude that these findings may explain why the treatment of stage 3 ONJ, namely surgery with success rate over 85%33,34, has been deemed to be more predictable and therefore yields more favourable outcomes than the treatment of stages 1 and 2.35
Denosumab is characterised by reversibility of its effect after treatment discontinuation, in contrast with bisphosphonates. This is in line with our findings since cessation of denosumab in two cases helped to improve their symptoms significantly.
MRONJ has been reported to occur after a mean administration period of 39.3 months and 35 infusions in oncology patients.23 It is interesting that all published cases of denosumab-related ONJ occurred early after commencement of therapy, independent of the number of previous administrations.36,37 In our experience, all patients developed MRONJ within the first 3 months of teeth extractions; well ahead of the reported period and number of administrations of denosumab.
Furthermore, all four cases have had extensive lytic lesions developed following removal of a single tooth. The common radiographic findings in all cases include:
- non-healing extraction socket
- areas of focal and diffuse sclerosis
- thickened lamina dura
- early sequestrum formation
- reactive periosteal bone
- osteolysis of cortical and spongious bone
These findings, although common in MRONJ cases, have had extensive bony involvement and rapid progression of ONJ, demonstrating a far more aggressive nature of the disease compared to that seen with BPs.
In our experience, not all patients are adequately informed of the risks and adverse events of denosumab therapy. This highlights the importance of educating patients and inter-professional communication regarding the prevention and best management of MRONJ cases. In one of the cases, the lack of patient education concerning denosumab side effects and the failure of inter-professional communication had a detrimental effect on the patient’s overall management and subsequently patient’s oral health.
Table 2 Important Points
|
Conclusion
We present our experience with denosumab-related ONJ from Sheffield Teaching Hospital’s NHS Trust. This case series should contribute to the existing sparse clinical literature on this topic. The pathogenesis, treatment and outcome of ONJ are complex and multifactorial. Patients treated with denosumab may be more prone to developing ONJ even without a precipitating dental event. ONJ may have a more aggressive profile and develop significantly earlier in patients receiving denosumab. Prevention of ONJ still remains the most important goal, and this is most directly accomplished by avoiding invasive dental procedures and establishing inter-professional communication.
|
Acknowledgements Radiographic reports used is courtesy of Mr Martin Payne, Consultant in Dental Radiology and Assessment and Casualty, Charles Clifford Dental Hospital, Wellesley Road, Sheffield S10 2SZ. Competing Interests None declared Author Details ELENA KYRIAKIDOU BDS MFDS RCSEd MClinDent MOral Surg RCS Eng University of Sheffield, School of Clinical Dentistry, 19 Claremont Crescent, Sheffield S10 2TA, UK. MOHAMED BADR BDS MSc PhD University of Sheffield, School of Clinical Dentistry, 19 Claremont Crescent, Sheffield S10 2TA, UK. SIMON ATKINS BDS MFDS RCSEd FDSRCS PhD University of Sheffield, School of Clinical Dentistry, 19 Claremont Crescent, Sheffield S10 2TA, UK. SHEELAH HARRISON BDS, FDSRCS, PhD University of Sheffield, School of Clinical Dentistry, 19 Claremont Crescent, Sheffield S10 2TA, UK. CORRESPONDENCE: ELENA KYRIAKIDOU University of Sheffield, School of Clinical Dentistry, 19 Claremont Crescent, Sheffield S10 2TA, UK. Email: e.kyriakidou@sheffield.ac.uk |
References
- Hofbauer LC, Rachner TD, Coleman RE, Jakob F. Endocrine aspects of bone metastases. The Lancet Diabetes & Endocrinology. 2014;2(6):500-12.
- Gralow JR, Biermann JS, Farooki A, Fornier MN, Gagel RF, Kumar RN, et al. NCCN Task Force Report: Bone Health in Cancer Care. Journal of the National Comprehensive Cancer Network : JNCCN. 2009;7 Suppl 3:S1-32; quiz S3-5.
- Lipton A, Uzzo R, Amato RJ, Ellis GK, Hakimian B, Roodman GD, et al. The Science and Practice of Bone Health in Oncology: Managing Bone Loss and Metastasis in Patients With Solid Tumors. Journal of the National Comprehensive Cancer Network : JNCCN. 2009;7(Suppl 7):S1-S30.
- Brown-Glaberman U, Stopeck AT. Impact of denosumab on bone mass in cancer patients. Clinical Pharmacology : Advances and Applications. 2013;5:117-29.
- Ruggiero. American Association of Oral and Maxillofacial Surgeons Position Paper on Medication-Related Osteonecrosis of the Jaw-2014 Update (vol 72, pg 1938, 2014). J Oral Maxil Surg. 2015;73(9):1879-.
- Borumandi F, Aghaloo T, Cascarini L, Gaggl A, Fasanmade K. Anti-resorptive Drugs and their Impact on Maxillofacial Bone among Cancer Patients. Anti-cancer agents in medicinal chemistry. 2015;15(6):736-43.
- Fedele S, Bedogni G, Scoletta M, Favia G, Colella G, Agrillo A, et al. Up to a quarter of patients with osteonecrosis of the jaw associated with antiresorptive agents remain undiagnosed. British Journal of Oral and Maxillofacial Surgery. 2015;53(1):13-7.
- Kulkarni R, Cymerman JA, Pick A, Patel, Sutton, Abdel G, et al. Antiresorptive related osteonecrosis of the Jaw Bone (ARONJ): A single Maxillofacial Unit Case series and Analysis. British Journal of Oral and Maxillofacial Surgery. 2014;52(8):e74.
- Barasch A, Cunha-Cruz J, Curro FA, Hujoel P, Sung AH, Vena D, et al. Risk factors for osteonecrosis of the jaws: a case-control study from the CONDOR dental PBRN. Journal of dental research. 2011;90(4):439-44.
- Sim I-W, Sanders KM, Seymour J, Ebeling PR. Declining Incidence of Antiresorptive Drug-Associated Osteonecrosis of the Jaw (ARONJ) in Patients with Cancer: The Importance of Oral Hygiene. Osteoporosis: Risk Factors and Complications of Therapy. p. PP22-3-PP-3.
- Kearns AE, Khosla S, Kostenuik PJ. Receptor Activator of Nuclear Factor κB Ligand and Osteoprotegerin Regulation of Bone Remodeling in Health and Disease. Endocrine Reviews. 2008;29(2):155-92.
- Van den Wyngaert T, Wouters K, Huizing MT, Vermorken JB. RANK ligand inhibition in bone metastatic cancer and risk of osteonecrosis of the jaw (ONJ): non bis in idem? Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2011;19(12):2035-40.
- Campisi G, Fedele S, Fusco V, Pizzo G, Di Fede O, Bedogni A. Epidemiology, clinical manifestations, risk reduction and treatment strategies of jaw osteonecrosis in cancer patients exposed to antiresorptive agents. Future oncology. 2014;10(2):257-75.
- O'Halloran M, Boyd NM, Smith A. Denosumab and osteonecrosis of the jaws – the pharmacology, pathogenesis and a report of two cases. Australian Dental Journal. 2014;59(4):516-9.
- Narayanan P. Denosumab: A comprehensive review. South Asian Journal of Cancer. 2013;2(4):272-7.
- Boonen S, Adachi JD, Man Z, Cummings SR, Lippuner K, Torring O, et al. Treatment with denosumab reduces the incidence of new vertebral and hip fractures in postmenopausal women at high risk. The Journal of clinical endocrinology and metabolism. 2011;96(6):1727-36.
- Epstein MS, Ephros HD, Epstein JB. Review of current literature and implications of RANKL inhibitors for oral health care providers. Or Surg or Med or Pa. 2013;116(6):E437-E42.
- FDA Approval for Denosumab. Available from: http://www.cancer.gov/about-cancer/treatment/drugs/fda-denosumab.
- Roelofs AJ, Coxon FP, Ebetino FH, Lundy MW, Henneman ZJ, Nancollas GH, et al. Fluorescent Risedronate Analogues Reveal Bisphosphonate Uptake by Bone Marrow Monocytes and Localization Around Osteocytes In Vivo. Journal of Bone and Mineral Research. 2010;25(3):606-16.
- Heymann D. Anti-RANKL therapy for bone tumours: Basic, pre-clinical and clinical evidences. Journal of Bone Oncology. 2012;1(1):2-11.
- Russell RGG, Watts NB, Ebetino FH, Rogers MJ. Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos Int. 2008;19(6):733-59.
- Ruggiero SL, Dodson TB, Fantasia J, Goodday R, Aghaloo T, Mehrotra B, et al. American Association of Oral and Maxillofacial Surgeons Position Paper on Medication-Related Osteonecrosis of the Jaw-2014 Update. J Oral Maxil Surg. 2014;72(10):1938-56.
- Khan AA, Morrison A, Hanley DA, Felsenberg D, McCauley LK, O'Ryan F, et al. Diagnosis and management of osteonecrosis of the jaw: a systematic review and international consensus. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2015;30(1):3-23.
- Epstein JB, Guneri P, Barasch A. Appropriate and necessary oral care for people with cancer: guidance to obtain the right oral and dental care at the right time. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2014;22(7):1981-8.
- O'Ryan FS, Khoury S, Liao W, Han MM, Hui RL, Baer D, et al. Intravenous Bisphosphonate-Related Osteonecrosis of the Jaw: Bone Scintigraphy as an Early Indicator. J Oral Maxil Surg. 2009;67(7):1363-72.
- Watters AL, Hansen HJ, Williams T, Chou JF, Riedel E, Halpern J, et al. Intravenous bisphosphonate–related osteonecrosis of the jaw: Long-term follow-up of 109 patients. Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology. 2013;115(2):192-200.
- Badros A, Terpos E, Katodritou E, Goloubeva O, Kastritis E, Verrou E, et al. Natural History of Osteonecrosis of the Jaw in Patients With Multiple Myeloma. Journal of Clinical Oncology. 2008;26(36):5904-9.
- Van den Wyngaert T, Claeys T, Huizing MT, Vermorken JB, Fossion E. Initial experience with conservative treatment in cancer patients with osteonecrosis of the jaw (ONJ) and predictors of outcome. Annals of Oncology. 2009;20(2):331-6.
- Saad F, Brown JE, Van Poznak C, Ibrahim T, Stemmer SM, Stopeck AT, et al. Incidence, risk factors, and outcomes of osteonecrosis of the jaw: integrated analysis from three blinded active-controlled phase III trials in cancer patients with bone metastases. Annals of Oncology. 2011.
- Allen MR, Ruggiero SL. A review of pharmaceutical agents and oral bone health: how osteonecrosis of the jaw has affected the field. The International journal of oral & maxillofacial implants. 2014;29(1):e45-57.
- Scoletta M, Arduino PG, Dalmasso P, Broccoletti R, Mozzati M. Treatment outcomes in patients with bisphosphonate-related osteonecrosis of the jaws: a prospective study. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics. 2010;110(1):46-53.
- Ruggiero SL, Dodson TB, Fantasia J, Goodday R, Aghaloo T, Mehrotra B, et al. American Association of Oral and Maxillofacial Surgeons Position Paper on Medication-Related Osteonecrosis of the Jaw—2014 Update. J Oral Maxil Surg.72(10):1938-56.
- Voss PJ, Joshi Oshero J, Kovalova-Müller A, Veigel Merino EA, Sauerbier S, Al-Jamali J, et al. Surgical treatment of bisphosphonate-associated osteonecrosis of the jaw: Technical report and follow up of 21 patients. Journal of Cranio-Maxillofacial Surgery. 2012;40(8):719-25.
- Pautke C, Bauer F, Otto S, Tischer T, Steiner T, Weitz J, et al. Fluorescence-Guided Bone Resection in Bisphosphonate-Related Osteonecrosis of the Jaws: First Clinical Results of a Prospective Pilot Study. J Oral Maxil Surg. 2011;69(1):84-91.
- Graziani F, Vescovi P, Campisi G, Favia G, Gabriele M, Gaeta GM, et al. Resective surgical approach shows a high performance in the management of advanced cases of bisphosphonate-related osteonecrosis of the jaws: a retrospective survey of 347 cases. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons. 2012;70(11):2501-7.
- Otto S, Baumann S, Ehrenfeld M, Pautke C. Successful surgical management of osteonecrosis of the jaw due to RANK-ligand inhibitor treatment using fluorescence guided bone resection. Journal of Cranio-Maxillofacial Surgery. 2013;41(7):694-8.
- Diz P, López-Cedrún JL, Arenaz J, Scully C. Denosumab-related osteonecrosis of the jaw. Journal of the American Dental Association. 2012;143(9):981-4.

The above article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.




