Cyclophosphamide and Doxorubicin-induced Acute Pancreatitis in a Patient with Breast Cancer
Vincent Bryan Salvador, Manpreet Singh, Philip Witek and Gay Peress
Cite this article as: BJMP 2014;7(3):a727
|
|
Abstract Predominantly occurring as a consequence of alcohol use or biliary stones, acute pancreatitis is rarely caused by chemotherapy. Lately, there have been increasing published reports and reviews of drug-induced pancreatitis from a wide array of antineoplastic drugs. We present a case of a patient recently diagnosed with Stage 3 breast cancer who was initially treated with cyclophosphamide and doxorubicin and subsequently developed acute pancreatitis, which recurred twice after a re-challenge with cyclophosphamide and epirubicin, a derivative of doxorubicin, given individually on two separate occasions. Acute pancreatitis reported in this case is defined by its clinical manifestations, biochemical evidence and imaging studies. To our knowledge, this is the first case of acute pancreatitis occurring in a patient with breast cancer associated with these chemotherapeutic agents. Keywords: acute pancreatitis, chemotherapy, cyclophosphamide, doxorubicin, drug-induced pancreatitisAbbreviations: CBC-complete blood count, FDA-Federal Drug Administration, NPO-nothing per os |
INTRODUCTION
Although it is well appreciated that pancreatitis is frequently secondary to biliary tract disease and alcohol abuse, it can also be caused by drugs, trauma and viral infections, or even be associated with metabolic and connective tissue disorders.1 Knowledge of the true incidence of drug-induced acute pancreatitis is dependent on the clinician’s ability to exclude other possible causes, and by promptly reporting the occurrence. Based on individual case reports and case control studies of drug-induced acute pancreatitis, the estimated overall incidence ranges from between 0.1 and 2% of pancreatitis cases.2,3 In particular, drug-induced acute pancreatitis is of mild severity and usually resolves without significant complications.4
Attempts have been made to categorize the risk of drugs causing acute pancreatitis. A previous classification system described by Mallory and Kern Jr. categorized drugs associated with acute pancreatitis as definite, probable, or possible.5 Trivedi et al. proposed a newer classification system for commonly used medications associated with drug-induced pancreatitis. Class I drugs are those medications with at least 20 reported cases of acute pancreatitis and at least one case with a positive rechallenge. Drugs with fewer than 20 but more than 10 reported cases of acute pancreatitis, with or without a positive rechallenge, are designated into Class II. While those medications with 10 or less reported cases, or unpublished reports in pharmaceutical or FDA files, are grouped into Class III.6
Acute pancreatitis as a result of either doxorubicin or cyclophosphamide, or a combination of both, or fluorouracil or epirubicin is a rare occurrence and has seldom been reported in the literature. Even the drug package labels registered with the FDA do not indicate the possible occurrence of pancreatitis. In this case report, we present a rare occurrence of drug-induced acute pancreatitis after the completion of the first cycle of the chemotherapy protocol involving cyclophosphamide and doxorubicin in a patient with stage 3 breast cancer, with recurrences of acute pancreatitis after re-challenging with cyclophosphamide and a derivative of doxorubicin, given individually on two separate occasions.
CASE PRESENTATION
A 58 year-old female presented to the emergency room with a one day history of severe, diffuse, deep-seated abdominal pain that radiated to her back, associated with nausea and vomiting, and was unrelieved despite the intake of NSAIDs. There was no reported fever, chills, diarrhea, dysuria, or antecedent trauma. Her medical history is notable for well-controlled hypertension, hyperlipidemia and hypothyroidism for which she takes amlodipine, atorvastatin and levothyroxine. She was recently diagnosed with left-sided breast cancer, Stage III, two months prior to admission and underwent a left modified radical mastectomy. Three days prior to the hospital visit, she was given her first cycle of chemotherapy with Doxorubicin 60 mg/m2 and Cyclophosphamide 600 mg/m2 along with Pegfilgrastim 6 mg and Fosaprepitant 150 mg. She is a former cigarette smoker, drinks alcohol infrequently, and denies illicit drug use. Her family history is unremarkable.
Physical examination revealed stable vital signs without a fever (36.6◦C). She had non-icteric sclerae and a dry oral mucosa. Chest exam revealed a well-healed left mastectomy scar and an infusaport located on the right anterior chest wall. Her breath sounds were clear bilaterally. Her heart sounds were normal. Her abdominal exam was significant for mild tenderness to palpation in the epigastric area without palpable masses, organomegaly or ascites. There was no evident ecchymosis observed. The extremities were warm to touch with intact and symmetrical pulses, and without bipedal edema.
Initial work-up revealed an elevated leukocyte count of 42,000 with 80% neutrophils and 17% band forms. Basic metabolic panel was normal except for mild hyponatremia of 129 mEq/L. Serum amylase and lipase were markedly elevated at 2802 units/L and >2000 units/L, respectively. Liver function panel was normal (Alk phos 63 U/L [ref range 30-115 U/L], GGT 21 U/L [ref range 3-40 U/L], total bilirubin 0.90 mg/dL [ref range 0-1.5 mg/dL]). The coagulation profile was within normal range. Imaging of the abdomen with a CAT scan with intravenous and oral contrast showed haziness in the pancreatic fat plane suggestive of pancreatic inflammation, with no gallstones, focal abscesses, hepatic masses, or biliary ductal dilatation (Figure 1). Right upper quadrant ultrasound was essentially normal (Figure 2).
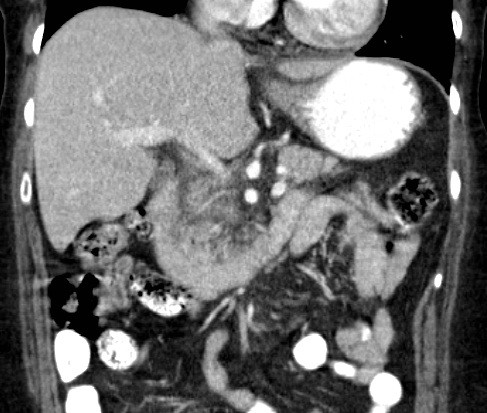
Figure 1. Coronal view of CT scan of abdomen and pelvis with IV and oral contrast showing haziness in the peripancreatic fat plane.
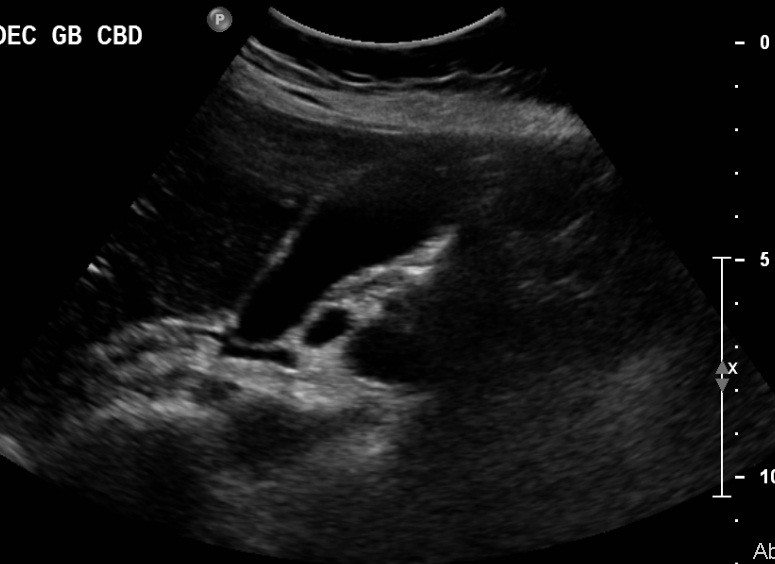
Figure 2. Sonogram of the right upper quadrant of the abdomen showing gallbladder devoid of gallstones and non-dilated common bile duct.
She was admitted to the medicine unit with the assessment of acute pancreatitis likely secondary to doxorubicin and cyclophosphamide. Intravenous fluid hydration with normal saline was initiated. She was kept NPO (nothing per os) and was started on empiric Imipenem and IV Esomeprazole. Her abdominal pain was controlled with intravenous morphine and her nausea with Ondansetron as needed. The serial basic chemistry panel was monitored and electrolyte deficits were replaced accordingly. Further work-up was performed to identify other possible etiologies of pancreatitis. The lipid panel was within normal limits (cholesterol 169 mg/dL [0-200 mg/dL], HDL 74 mg/dL, LDL 71 mg/dL and triglycerides 54 mg/dL [0-150 mg/dL]). The serum calcium levels remained within the normal range throughout her hospital stay. An abdominal sonogram demonstrated absence of gallstones or dilatation of the common bile duct, with a normal appearing liver parenchyma and pancreas. During her stay in the medicine unit, the patient’s abdominal pain improved and she was gradually started on an oral diet, which she tolerated well. Her serum electrolytes remained stable, while her serial CBC revealed progressively decreasing trends in leukocytes, hemoglobin, hematocrit, and platelet count, findings which were attributed to her prior chemotherapy. Repeat serum amylase and lipase both trended downward. The patient was discharged with follow up in the Oncology clinic. A month later, she was started on another chemotherapy regimen that consisted of weekly administration of Paclitaxel 80 mg/m2 which, over the next two months, the patient completed without any complications. Then, after explaining the risks of recurrent pancreatitis, the patient consented to have a trial of cyclophosphamide 500 mg/m2 along with fluorouracil 500 mg/m2. Five days after receiving the chemotherapy, the patient developed acute pancreatitis which was attributed to cyclophosphamide. She again made a full recovery at that time. Three weeks later, her chemotherapy regimen was again changed, to epirubicin 90 mg/m2 and fluorouracil. Four days after receiving this regimen, she again, for the third time, had a recurrence of acute pancreatitis. At this time, a repeat abdominal sonogram revealed a 4mm echogenic focus adherent to the anterior gallbladder wall with a comet tail sign, suggestive of cholesterol crystals lodged within Rokitansky-Aschoff sinuses of the gallbladder wall. There were no visible gallstones. A subsequent MRI of the abdomen with contrast revealed a small rounded hypointensity in the dependent portion of the gallbladder wall that was suggestive of a gallstone, however, there was no biliary obstruction, choledocholithiasis or an obstructing pancreatic mass. At this point, chemotherapy was stopped and anastrozole along with radiation therapy was initiated. The patient continues to be followed regularly and has had no recurrence of pancreatitis since her last episode.
DISCUSSION
The case presented described the development of acute pancreatitis in a patient with breast cancer three days after receiving the chemotherapy regimen consisting of cyclophosphamide and doxorubicin. After re-challenging the patient with cyclophosphamide, and again a few weeks later with a derivative of doxorubicin, epirubicin, acute pancreatitis recurred on each occasion. Despite the presence of cholelithiasis detected on the abdominal MRI, the temporal presentation of acute pancreatitis after chemotherapy exposure is highly suggestive of the role these chemotherapeutic agents played in triggering these three acute attacks. Acute pancreatitis was diagnosed based on the clinical suspicion and symptoms suggestive of the acute pancreatitis and was supported by the marked elevation in serum amylase and lipase, as well as, the radiologic evidence of pancreatic inflammation, both of which are markers of acute pancreatitis.
Chemotherapy-induced acute pancreatitis involving cyclophosphamide and doxorubicin either alone or in combination, is quite rare that even the drug labels registered with the FDA do not indicate acute pancreatitis as one of the possible complications. This scenario highlights the importance of drug surveillance and prompt reporting in order to maintain a credible drug safety database.
Though the drug latency, which is the interval between the initial exposure to the drug and the development of acute pancreatitis, differs variably, the present case is considered to have an intermediate latency (1-30 days). Other drugs may have short (< 24 hours) or long (>30 days) latency periods. Examples of drugs with short latency are acetaminophen, codeine, erythromycin and propofol. Intermediate latency drugs include L-asparaginase, pentamidine and stibugluconate. Drugs with long latency are estrogen, tamoxifen, valproate and dideoxyinosine.7
Based on the revised classification of Badalov et al, the combination of cyclophosphamide and doxorubicin is classified as Class IV drugs, which have the weakest association with acute pancreatitis due to limited information and the lack of adequate detailed case reports. Fluorouracil, which has been known to cause a gastrointestinal ulcer, is also categorized as a Class IV drug, while epirubicin, which is derived from doxorubicin, has not been classified, as it has not been reported before to cause acute pancreatitis. In implicating drugs in the etiology of acute pancreatitis, two conditions must be considered to weigh the strength of the association between the causality and the disease process, namely: a positive rechallenge test resulting in the recurrence of pancreatitis and a similar latency period between the drug exposure and development of the disease.7
The combination of drugs rather than a single agent was implicated for drug-induced pancreatitis in a previous case report that described the development of acute pancreatitis shortly after the second cycle of the chemotherapy regimen composed of cyclophosphamide, doxorubicin, and vincristine in a patient with mediastinal immunoblastic lymphoma. The pancreatitis episode resolved over 48 hours without complications.8
Another case was described in a patient with breast cancer developing acute pancreatitis four days after the third cycle of chemotherapy, which involved docetaxel and carboplatin.9
Toprak et al. reported the occurrence of acute pancreatitis in a patient with multiple myeloma after the initial treatment with the triple regimen chemotherapy protocol consisting of vincristine, doxorubicin, and dexamethasone. In this case report, symptoms suggestive of acute pancreatitis started to manifest on the first day of the treatment, with resolution following discontinuation of the drugs.10
Other antineoplastic agents for breast cancer associated with drug-induced pancreatitis are alemtuzumab, trastuzumab and tamoxifen. Extended use of these medications may cause chronic pancreatitis as a result of their causing repeated clinical or subclinical episodes of acute pancreatitis.6 Most cases of drug-induced pancreatitis follow a mild clinical course.7
In a retrospective study involving 1613 patients diagnosed with acute pancreatitis in a gastroenterology center, the incidence of drug-induced pancreatitis had been reported in 1.4% of patients treated for acute pancreatitis. It has been observed that a higher incidence of drug-induced acute pancreatitis occurs in elderly or pediatric patients, and in those patients with inflammatory bowel disease or AIDS.11
The pathophysiology behind drug-induced pancreatic injury remains unclear. Potential mechanisms underlying such pancreatic injury might be related to drug hepatotoxicity which can be secondary to intrinsic toxicity of the drugs affecting the tissue, or due to an idiosyncratic reaction. In the vast majority of the cases, an idiosyncratic reaction could be the main pathway for tissue injury through a hypersensitivity reaction or production of toxic intermediate metabolites. Idiosyncratic reactions have a longer latency period of months to years before the onset of pancreatitis while the onset of hypersensitivity reactions is earlier (i.e. 1-6 weeks).7
CONCLUSION
Due to a variable latent period between the initial drug exposure and the onset of clinical symptoms, drug-induced pancreatitis must remain as a differential diagnosis in patients receiving chemotherapy regimens and presenting with the constellation of symptoms typical of acute pancreatitis. Due to the unclear pathogenesis of chemotherapy-induced pancreatitis, post-marketing surveillance and adverse drug reporting are paramount in elucidating the effect these drugs have on the pancreas.
|
Competing Interests None declared Author Details VINCENT BRYAN SALVADOR, M.D. Department of Medicine, Icahn School of Medicine at Mt. Sinai/Queens Hospital Center, 82-68 164th Street, Jamaica, New York, USA 11432. MANPREET SINGH, M.D. Department of Medicine, Icahn School of Medicine at Mt. Sinai/Queens Hospital Center, 82-68 164th Street, Jamaica, New York, USA 11432. PHILIP WITEK, M.D. Department of Medicine, Icahn School of Medicine at Mt. Sinai/Queens Hospital Center, 82-68 164th Street, Jamaica, New York, USA 11432. GAY PERESS, M.D. Department of Medicine, Icahn School of Medicine at Mt. Sinai/Queens Hospital Center, 82-68 164th Street, Jamaica, New York, USA 11432. CORRESPONDENCE: VINCENT BRYAN SALVADOR, M.D. Department of Medicine, Icahn School of Medicine at Mt. Sinai/Queens Hospital Center. 82-68 164th Street, Jamaica, New York, USA 11432. Email: docvincesalvador@aol.com |
References
- Sakorafas GH & Tsiotou AG. Etiology and pathogenesis of acute pancreatitis: current concepts. J Clin Gastroenterol. 2000;30:343-356.
- Nitsche CJ, Jamieson N, Lerch MM et al. Drug induced pancreatitis. Best Pract Res Clin Gastroenterol.2010;24(2):143-145.
- Wilmink T & Frick TW. Drug induced pancreatitis. Drug Safety. 1996;14:406-423.
- Tonsi AF, Bacchion M, Crippa S, Malleo G & Bassi C. Acute pancreatitis at the beginning of the 21st century: The state of the art. World J Gastroenterol 2009; 15(24):2945-2959.
- Mallory A & Kern F Jr. Drug-induced Pancreatitis: A Critical Review. Gastroenterol. 1980;78:813-820.
- Trivedi CD & Pitchumoni CS. Drug-Induced Pancreatitis: An Update. J Clin Gastroenterol. 2005;39:709-716.
- Badalov N, Baradarian R, Iswara K et al. Drug-Induced Acute Pancreatitis: An Evidence-Based Review. Clin Gastroenterol Hepatol. 2007;5:648-661.
- Puckett JB, William B & McFarland JA. Pancreatitis and Cancer Chemotherapy. Ann Intern Med. 1982;97(3):453.
- Singh V, Devata S & Cheng YC. Carboplatin and docetaxel-induced acute pancreatitis: brief report. Int J Clin Oncol. 2010;15:642-644.
- Toprak SK, Ocal S, Erismis B et al. Acute Pancreatitis Following VAD Chemotherapy Combination Consisting of Vincristine, Doxorubicin, and Dexamethasone in a Newly Diagnosed Multiple Myeloma Patient: A Case Report. The Internet Journal of Oncology. 2012; 8(2). Accessed at: http://archive.ispub.com/journal/the-internet-journal-of-oncology/volume-8-issue-2/acute-pancreatitis-following-vad-chemotherapy-combination-consisting-of-vincristine-doxorubicin-and-dexamethasone-in-a-newly-diagnosed-multiple-myeloma-patient-a-case-report.html#sthash.BFCHZGAr.mjxZj1fi.dpuf. Accessed on: 18 October 2013.
- Lankisch PG, Droge M & Gottesleben F. Drug induced Acute Pancreatitis: Incidence and Severity. Gut. 1995;37:565-567.

The above article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.




