Clozapine initiation in crisis teams
Carlos Gonzalez, Kalyani Kodimela, Amanda Poynton
Cite this article as: BJMP 2013;6(3):a624
|
|
Abstract Aims: Our aim was to investigate the practicalities and success rate of clozapine titration in the community in a large sample of patients referred to three crisis teams in a defined geographical area. Methods: We collected data retrospectively of all the referrals for clozapine initiation to three crisis teams across Manchester during a three year period. Results: Out of a total of 6542 referrals, 66 were for clozapine initiation. From these referrals only 54 patients started clozapine, as the others declined the treatment. After commencing clozapine, a total of 46 patients (86.2%) completed the titration successfully. The main reason for discontinuing the clozapine titration in the community was withdrawal of patients’ consent. Only one patient was unable to physically tolerate the titration and was admitted to hospital. Conclusions: Clozapine can be safely started in the community. Patients’ adherence to the treatment and to the physical monitoring is the key element of a successful outcome. Crisis teams are in an ideal position to support patients undergoing initiation of clozapine at home. |
Introduction
Clozapine has shown to have superior efficacy compared to other antipsychotics and is the drug of choice for treatment-resistant schizophrenia. 1 However there is evidence that this treatment is actually under-prescribed. 2 Clozapine requires careful monitoring during the initial titration period. In the UK, this has originally been done in hospital settings to follow the manufacturer’s recommendations because of the risks of hypotension, excessive sedation and fits. Starting clozapine in a hospital setting ceased to be a mandatory regulatory requirement in the UK when the Summary of Product Characteristics was harmonised across Europe following an opinion and recommendation issued on the 12th of November 2002 by the Committee for Proprietary Medical Products of the European Medicines Agency. 3 Despite this happening several years ago, there is little information published about the practicality of successfully commencing clozapine in the community, with previous studies ranging from a single case report 4 to a few small case series of patients 5-8. Our study aimed to examine this practice in a larger sample to highlight the advantages and difficulties of initiating clozapine in the community.
Method
The Central Manchester day hospital was established in 1985, with a focus on acute psychiatric treatment as an alternative to in-patient care. From March 1997, the acute day hospital in Central Manchester was extended to 24 hours, seven days a week, adopting the name of the Home Option Service, focussing on flexible individualised care delivered at patient’s home or team base according to patient choice. 9 In 2007 as part of implementation of new teams across the city to comply with NHS policy guidance 10 the Home Option service developed further to become the crisis resolution and home treatment team (CRHT) for central Manchester, whilst CRHTs were set up de novo in North, and South Manchester, thereby, providing acute community psychiatric care to a metropolitan area of about 500,000 people.
This study describes a large case series of patients referred for clozapine titration in the community to these teams during a three year period. We collected data retrospectively from April 2007 to April 2010 of all the referrals to the three crisis teams, which have assumed the responsibility of providing the service of initiating clozapine in the community. The teams followed the Trust protocol for non-inpatient clozapine titration, which includes recommended monitoring parameters, dosing schedule and algorithms for the management of complications. This protocol is in essence similar to established guidelines. 4,5, 11
Statistical analysis was done using SPSS version 15 for Windows. Comparisons were made using the Student t-test, non parametric tests or the Chi-Square test according to the type of data.
Results
There were 6542 referrals to the crisis teams and 66 of those were related to clozapine initiation. Out of these, 36 were for a first time titration and 30 were referred for re-titration. The latter group were patients previously taking clozapine but who had discontinued it abruptly for a period longer than 48 hours. The reasons for stopping clozapine in those cases were lack of adherence (n=21), a supplying difficulty (n=5) and medical complications (n=4), such as neutropenia, collapse secondary to dehydration, or undergoing surgery. Two of the patients in the re-titration group restarted clozapine in hospital but were discharged early to continue the titration in the community under the care of the crisis team. Six patients in the titration group were initially referred to the crisis team for stabilisation of their mental state following a crisis; however, during the course of this intervention it was decided to start them on clozapine as they showed poor response to other antipsychotic trials.
Fig. 1 - Referrals, number of patients starting clozapine and drop-outs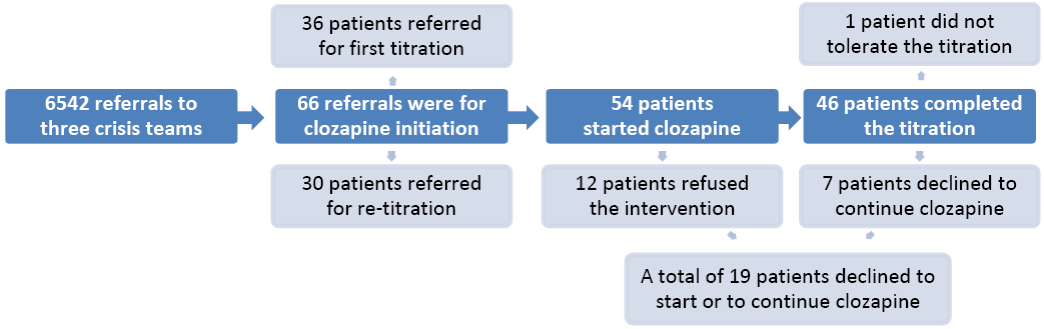
The characteristics of the sample are presented in Table 1. The majority of patients were single white males, with a diagnosis of schizophrenia and a mean age of 38.8 years (standard deviation = 9.2). The flowchart in Figure 1 outlines the number of referrals, titrations, and reasons for stopping. Clozapine titration commenced in 54 cases (81.8% of referrals), the other 12 patients refused this treatment. Out of the patients who refused treatment, 8 were severely mentally unwell and were admitted to hospital compulsorily under the Mental Health Act. There were 46 (85.2%) patients who successfully completed the community titration. The attrition rate of 14.8% (8 cases) was due to 7 patients withdrawing their consent and one patient who was unable to tolerate the titration. This person was admitted to hospital with hypotension and vomiting. The other 7 patients withdrew their consent for the following reasons: lack of adherence (n=2), deterioration in mental state (n=1), refusal to continue with the physical monitoring (n=1), lack of motivation (n=1), and reluctance to continue due to side-effects (n=2). The mean final dose of clozapine was 309.1 mg (s.d. - 75.1 mg). The mean duration of titration was 34.6 days (s.d. - 20.3) and the mean length of admission to the crisis team was 45.9 days (s.d. - 39.5). The median waiting time for crisis team intervention after the referral was 2 days (range 140 days). The median waiting time to start clozapine was 7 days (range 217 days) from the point of referral.
Table 1. Sample characteristics
| Total | ||
| Age in years, mean (s.d.) | 38.8 | (9.2) |
| Gender, n (%) | ||
| Male | 45 | (68.2) |
| Female | 21 | (31.8) |
| Ethnicity, n (%) | ||
| White | 45 | (68.2) |
| Black | 14 | (21.2) |
| Asian | 3 | (4.5) |
| Other | 4 | (6.1) |
| Marital Status, n (%) | ||
| Single | 54 | (81.8) |
| Married or cohabiting | 5 | (7.6) |
| Separated or divorced | 6 | (9.1) |
| Widowed | 1 | (1.5) |
| Diagnosis, n (%) | ||
| Schizophrenia | 54 | (81.8) |
| Schizoaffective disorder | 8 | (12.1) |
| Bipolar affective disorder | 1 | (1.5) |
| Other | 3 | (4.5) |
| Crisis Team, n (%) | ||
| North | 21 | (31.8) |
| Central | 31 | (47.0) |
| South | 14 | (21.2) |
| Days waiting to crisis team intervention | ||
| Mean (standard deviation) | 9.5 | (25.6) |
| Median (range) | 2 | (140) |
| Days waiting to start clozapine | ||
| Mean (standard deviation) | 23.1 | (40.9) |
| Median (range) | 7 | (217) |
| Days taken to complete the titration | ||
| Mean (standard deviation) | 34.6 | (20.3) |
| Median (range) | 28 | (101) |
| Days under the care of the crisis team | ||
| Mean (standard deviation) | 45.9 | (39.5) |
| Median (range) | 34 | (235) |
| Final dose in mg | ||
| Mean (standard deviation) | 309.1 | (75.1) |
There were few significant differences between the group of patients starting clozapine for the first time (titration) and those restarting it following a treatment break (re-titration). There was a shorter wait for patients in the re-titration group to recommence clozapine from the time of referral to the service (median=6 days, range = 41 days), compared to those starting clozapine for the first time (median=13 days, range= 217 days). This difference was statistically significant (Mann Whitney U= 201.5, z=-2.529, p=0.01). Patients with first titration on clozapine reached a lower final dose (mean=288 mg, s.d.=50 mg), compared to those having re-titration (mean dose =340 mg, s.d.=94 mg). The mean difference of 52.7 mg (95% C.I. 8.7 to 96.8) between these two groups was significant (t test=-2.178, d.f 42, p=0.02). In terms of ethnicity, patients in the initial titration group were more likely to be Caucasian (n=30, 83%), whereas only half of the patients in the re-titration group were Caucasian (n=15, 50%). This difference was statistically significant (Chi-square with continuity correction = 6.915, df = 1, p=0.009).
There were also significant differences in the distribution of titrations and re-titrations across the three crisis teams. The Central Team dealt with more re-titrations (n=23) than the North (n=4) and the South (n=3) teams. Conversely, the Central Team had fewer patients referred for initial titration (n=8), compared to the North (n=17) and the South (n=11) teams. These differences were significant (Chi-square=19.493, df=2, p<0.0001). Another difference between the teams was the duration of clozapine titration, with the South team taking shorter time (mean=24.15 days, s.d.=7.151), compared to the North (mean=29.5 days, s.d=16.342) and the Central team (mean =43.67 days, s.d.= 23.797). This difference was statistically significant (Kruskal-Wallis chi-square=8.823, d.f.=2, p=0.0121).
No significant differences were found between teams and titration or re-titration groups in terms of patient’s diagnosis, gender, marital status, age, rate of accepted referrals, proportion of successfully finished titrations or waiting time to crisis team intervention.
With regards to adverse events, most patients experienced transient tachycardia (n=30, 55.5%). Other side-effects were excessive salivation (n= 15), hypotension (n= 13), sedation (n=10), hyperthermia (n=8), dizziness (n=6), constipation (n=6), hypertension (n=5), headaches (n=4), nausea (n=2), and heartburn (n=2). Less common adverse events (n=1) were syncope, seizures, transient neutropenia, atrial fibrillation, blurred vision, swelling of the arms , acute dystonic reaction, nocturnal incontinence, exacerbation of asthma, diabetes, erectile dysfunction and delayed ejaculation. Only the patient who developed syncope, which was associated with vomiting and severe hypotension, had to be advised to stop the treatment in the community and was admitted to hospital. For the rest of the patients, the other reported adverse events did not impede the successful completion of clozapine titration in the community.
In terms of longer term outcomes, a total of 50 patients (75.8% of the total sample) were still taking clozapine at the time the data was collected. This is after a median 337 days (range 824 days) from being referred to the crisis team. The majority of patients (n=14, 21.2%) who were not on clozapine had chosen to discontinue the treatment. One patient had died, but the cause of death was not related to clozapine treatment. One patient had developed neutropenia and needed to discontinue clozapine for this reason. Out of the 46 patients who successfully completed the titration, 40 (86.96%) were still continuing clozapine at the time we collected the data. This is after a median 365.5 days (range 824 days) after they commenced clozapine in the community.
Discussion
The results of this study confirm that clozapine can be safely and successfully started in the community. Comparing this to published evidence, we found only one case report 4 and a small study 5,6 previously conducted in the UK. O’Brien et al. 5,6 initially considered 26 patients in their study; however, only 14 patients started clozapine in the community as the rest were considered too unwell and were admitted to hospital. One patient refused daily access and therefore only 13 patients completed the titration. The side effects reported in this study were minor, including sedation in 5 cases, dizziness in 4 patients, hypotension in two and nausea and vomiting once. Compared to our results, O’Brien et al. described a larger proportion of patients needing to be admitted to hospital for clozapine titration.
We found two published studies 7,8 regarding clozapine community titration that were conducted in the United States. The first study included 47 patients who started clozapine in a partial hospitalisation program. Adverse reactions here were common. Patients were titrated much more quickly than in our report, (i.e. to 350 mg over 2 weeks), which might explain the higher incidence of side effects reported, including drowsiness (93.6%), hypersalivation (93.6%), constipation (89.4%), weight gain (72.3%) and tachycardia (57.4%). However no patient discontinued clozapine, and the potentially serious complications were much less frequent, including 3 cases (6%) of seizures and 2 of leukopenia. The other study 8 conducted in the US demonstrated some evidence of cost savings associated with decreased hospitalisation in 28 patients who started clozapine on an outpatient basis.
Johnson et al. 7 discuss in their report that the reluctance to start clozapine outside inpatient settings may be due partly to the potential adverse reactions, but also to clinicians’ fears of making mistakes, avoidance of additional duties, and anticipation of difficulties in patients with a history of non-adherence to treatment. The results of our study support a careful approach to starting clozapine at home in this latter group of patients, as they represented the bulk of cases not achieving the intended outcome of a successful community clozapine titration. However, our study confirms that other reasons to deny a patient the opportunity to start clozapine at home, such as potential adverse events, are hardly justified.
The general advantages of community psychiatric care as opposed to inpatient treatment have been described elsewhere 9. These include accessibility, flexibility and user satisfaction. Treating patients in their own homes avoids the stigma of hospital admission, prevents the breakdown of important social networks and avoids disruption to patients' benefits. A recent Cochrane review 12 found that crisis/home care reduces the number of people disengaging early, reduces family burden, and is a more satisfactory form of care for both patients and families. Some patients who might have been reluctant to start clozapine if they had to be admitted to hospital can therefore benefit from starting this treatment at home supported by crisis teams.
Although a detailed cost-benefit evaluation of this service was not undertaken, it is fair to assume that the costs associated with titrating clozapine at home would be significantly lower than those associated with in-patient care, as demonstrated in previous studies. 8,12
In summary, clozapine can be safely started in the community, but has to be carefully monitored. Patients’ adherence to the treatment and to the physical monitoring requirements is the key element to a successful outcome. Crisis teams are in an ideal position to support patients undergoing initiation of clozapine at home, although this specific role was not originally identified in policy guidance.10 The results of this multi-site study are encouraging and can be applicable to other crisis or community teams nationally.
|
Competing Interests None declared Author Details CARLOS GONZALEZ, LMS, MRCPsych, Consultant in General Adult Psychiatry, East London NHS Foundation Trust, Rehabilitation and Recovery Team, East Ham Memorial Building, Shrewsbury Road, East Ham, London, UK. CORRESPONDENCE: CARLOS GONZALEZ, Rehabilitation and Recovery Team, East Ham Memorial Building, Shrewsbury Road, East Ham, London, E7 8QR Email: doctorcarlosgonzalez@gmail.com |
References
- Wahlbeck K, Cheine M, Essali MA. Clozapine versus typical neuroleptic medication for Schizophrenia. Cochrane Database Sys Rev 2000; (2): CD000059
- Duggan A, Warner J, Knapp M, et al. Modelling the impact of clozapine on suicide in patients with treatment resistant schizophrenia in the UK. Br J Psychiatry 2003; 182: 505-8
- European Medicines Agency (EMEA). Summary information on referral opinion following arbitration pursuant to Article 30 of Council Directive 2001/83/EC for Leponex and associated names International Non-Proprietary Name (INN): clozapine: Background information and Annex III. EMEA, 2002. http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Leponex_30/WC500010966.pdf
- Lovett L. Initiation of clozapine treatment at home. Prog Neurol Psychiatry 2004; 8:19-21
- O’Brien A. Starting clozapine in the community: a UK perspective, CNS Drugs 2004; 18 (13): 845-852
- O’Brien A, Firn M. Clozapine initiation in the community. Psychiatr Bulletin 2002; 26: 339-341
- Johnson CG, Littrell KH, Magill AN. Starting clozapine in a partial hospitalisation program. Hospital and Community Psychiatry 1994; 45:264-268
- Luchins DJ, Hanrahan P, Shinderman M, Lagios L, Fichtner CG. Initiating clozapine treatment in the outpatient clinic: service utilisation and cost trends. Psychiatric Services 1998; 49:1034-1038
- Judy Harrison, Amanda Poynton, John Marshall, Richard Gater, and Francis Creed.Open all hours: extending the role of the psychiatric day hospital. Psychiatr Bulletin, Jul 1999; 23: 400 - 404.
- Department of Health. The Mental Health Policy Implementation Guide: Crisis Resolution/Home Treatment Teams. Department of Health, 2001
- Taylor D, Paton C, Kapur Shitij. The Maudsley Prescribing Guidelines. 10th Ed. Informa Health Care; 2009
- Irving Claire B, Adams Clive E, Rice Karl. Crisis intervention for people with severe mental illnesses. Cochrane Database Sys Rev: 2006 Issue 4

The above article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.




