Cholestatic hepatitis: An unusual presentation of lisinopril induced hepatotoxicity
Gurpinder Singh, Amit Kachalia, Jaspreet Kaur, Kinjal Kachalia, Shaojun Liu and Vincent Rizzo
Cite this article as: BJMP 2014;7(3):a721
|
|
Abstract Previously published case reports have shown direct hepatocellular injury as the mechanism for lisinopril induced hepatotoxicity. We report case of a 47 year old female who presented with jaundice, diagnosed as cholestatic hepatitis;two years after initiation of lisinopril. Extensive work up was negative for other causes of hepatitis. Liver biopsy showed portal inflammation by lymphocytes without centrilobular necrosis, which is similar to earlier case reports. Discontinuation of angiotensin converting enzyme inhibitors (ACE-I) usually leads to normalization of liver enzymes, however continuation or re-initiation can be potentially fatal. This is the first reported case of lisinopril induced hepatotoxicity via cholestatic mechanism. Some reports hypothesize a metabolic idiosyncratic reaction as the molecular mechanism but currently there is no validated literature. This case highlights the need for further research to explore mechanisms for ACE-I mediated hepatotoxicity and to create awareness amongst physicians to consider ACE-I as an etiology for drug induced liver injury. Keywords: Angiotensin coverting enzyme inhibitor, Hepatotoxicity, Cholestatic hepatitis, LisinoprilAbbreviations: ACE-I: Angiotensin converting enzyme inhibitors; DILI: Drug induced liver injury; AST: Aspartate transaminase; ALT: Alanine transaminase; GGT: Gamma-glutamyl transferase; ALP: Alkaline phosphatase; ANA: Anti nuclear antibody; AMA: Anti mitochondrial antibody; ANCA: Anti-neutrophil cytoplasmic antibody; CKD: Chronic kidney disease |
Introduction:
Various classes of medications have been known to cause drug induced liver injury (DILI), however not much literature has been published regarding angiotensin converting enzyme inhibitors (ACE-I) causing DILI. Recent years have seen tremendous increases in ACE-I prescriptions for coronary artery disease, diabetic nephropathy and hypertension. We report the first case of lisinopril induced hepatitis via a cholestatic mechanism.
Case:
A 47 year old female with history of diabetes mellitus type 2, hypertension, chronic kidney disease (CKD)stage III, non-obstructive coronary artery disease was admitted with complains of generalized weakness, lack of appetite, yellow discoloration of skin and eyes, dark urine and white stools for 1 week prior to admission. She denied history of alcohol abuse, past liver disease, illicit drug use, recent sick contacts, fever, chills, travel. Current patient medications included lisinopril, pioglitazone, furosemide, atenolol, metformin and detemir. Patient was started on these medications about 2 years prior to admission. Patient received enalapril for 5 months before switching to lisinopril about 2 years prior to presentation.
Physical examination was positive for icteric sclera, icteric skin; negative for spider nevi, palmar erythema and asterixis. Exam did not reveal hepatomegaly or splenomegaly. Labs showed hemoglobin 8.7 gm/dl, normal white count and platelet, normal C-reactive protein, alkaline phosphatase (ALP) 750 U/L, aspartate transaminase (AST) 169 U/L, alanine transaminase (ALT) 210 U/L, gamma-glutamyl transferase (GGT) 813 U/L, total bilirubin 13.4mg/dl with conjugated fraction 7.7mg/dl, ammonia level 64. Prior to initiation of lisinopril ALP was 87 U/L, GGT 53 U/L, with AST18 U/L, ALT 11 U/L and normal bilirubin fractions. Hepatitis A, B, C and D serologies were negative. Serum acetaminophen level was normal. Anti nuclear antibody (ANA), anti- mitochondrial antibody (AMA), anti-endomysial antibody, c-anti-neutrophil cytoplasmic antibody (ANCA), p-ANCA was negative. Anti smooth muscle antibody was weakly positive in titre of 1: 40. Creatine kinase, ceruloplasmin and alpha -1 antitrypsin level were normal. Quantiferon gold was negative. Lipid panel was deranged with cholesterol level 1017 and low density lipoprotein 1006, triglycerides 255.
Ultrasonography and magnetic resonance imaging abdomen showed hepatomegaly 17.5cms but was negative for fatty infiltration of liver, stones, cirrhotic features or dilation of biliary tree. Liver biopsy was done which showed mild portal chronic hepatitis with lymphocytic infiltration (Fig: 1), cholestasis (Fig: 2), mild portal fibrosis (Fig: 3), negative for bile duct damage (Fig: 4), negative for cytoplasmic inclusion. Congo red stain was negative for amyloid.
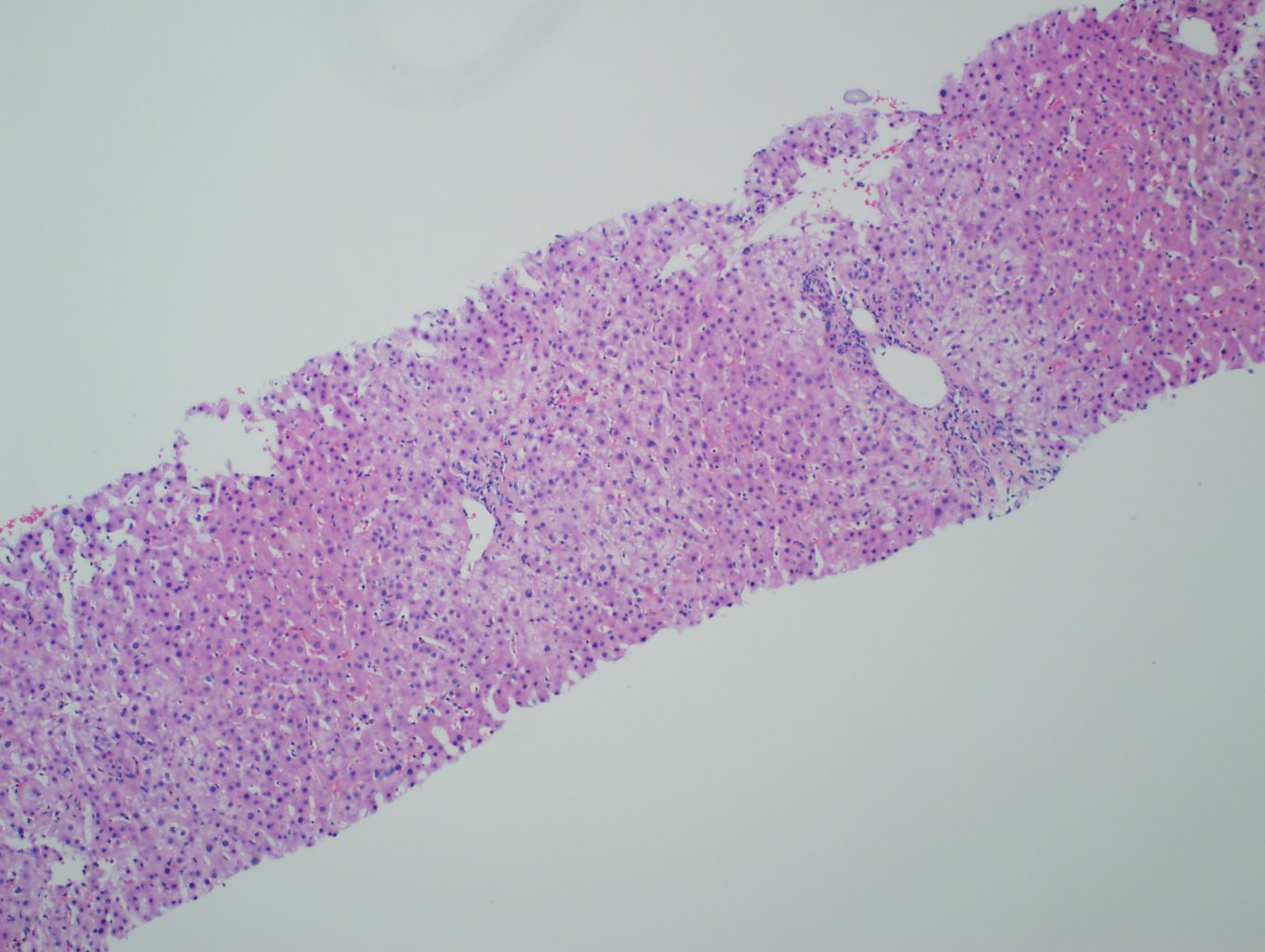
Figure 1: Mild hepatitis with portal tract lymphocytic infiltration.
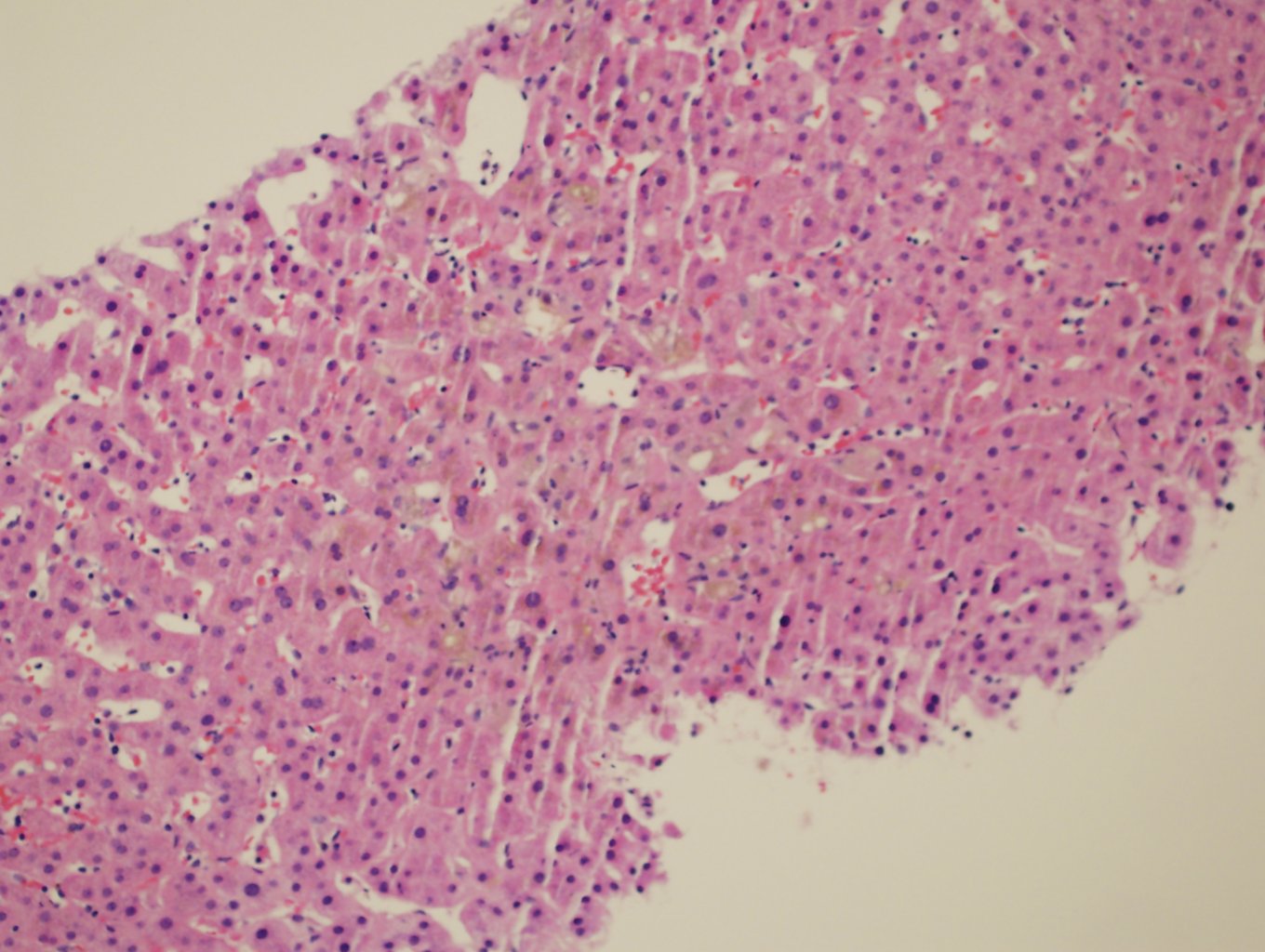
Figure 2: Cholestasis.
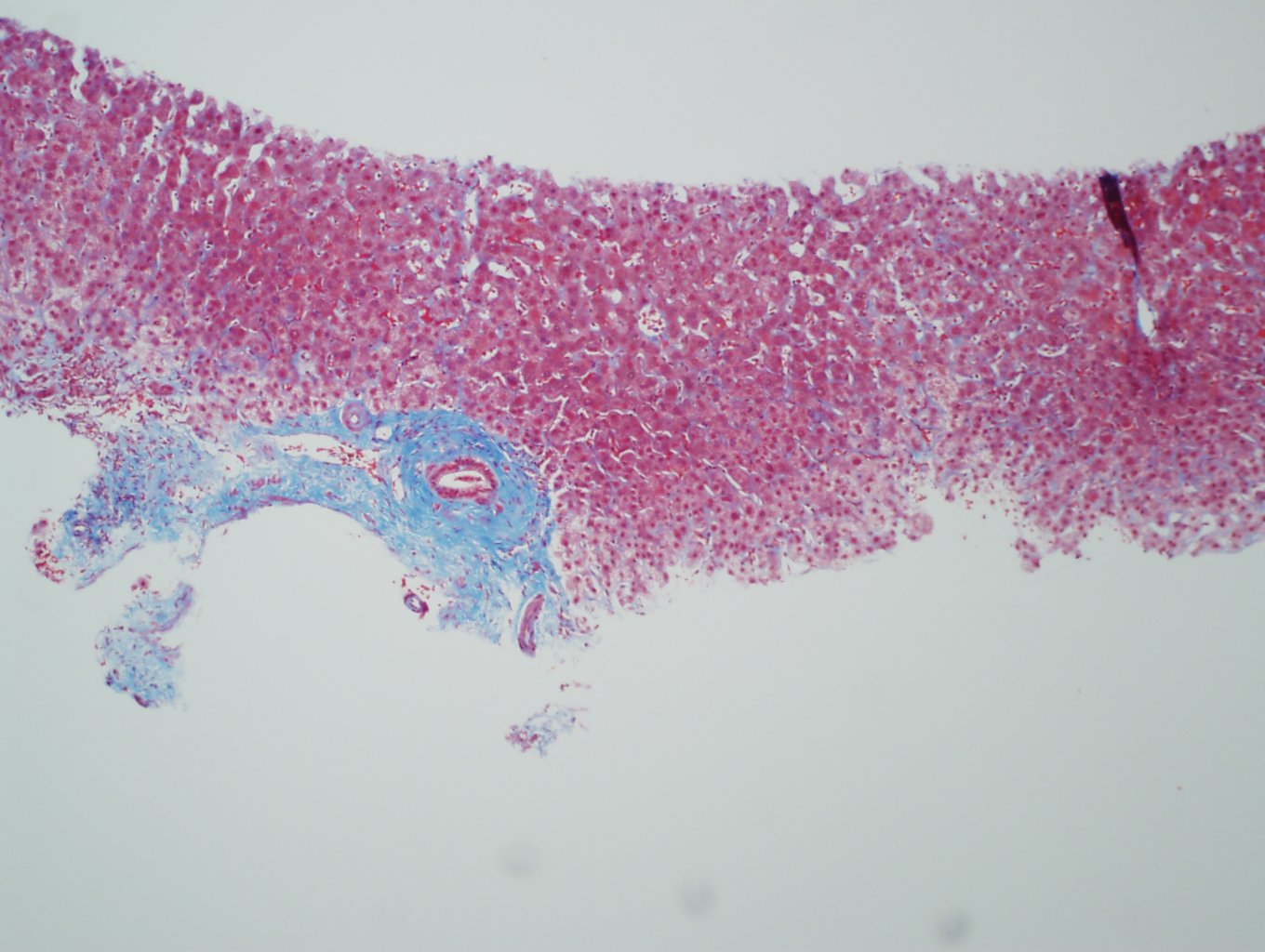
Figure 3: Trichrome stain showing portal tract fibrosis.
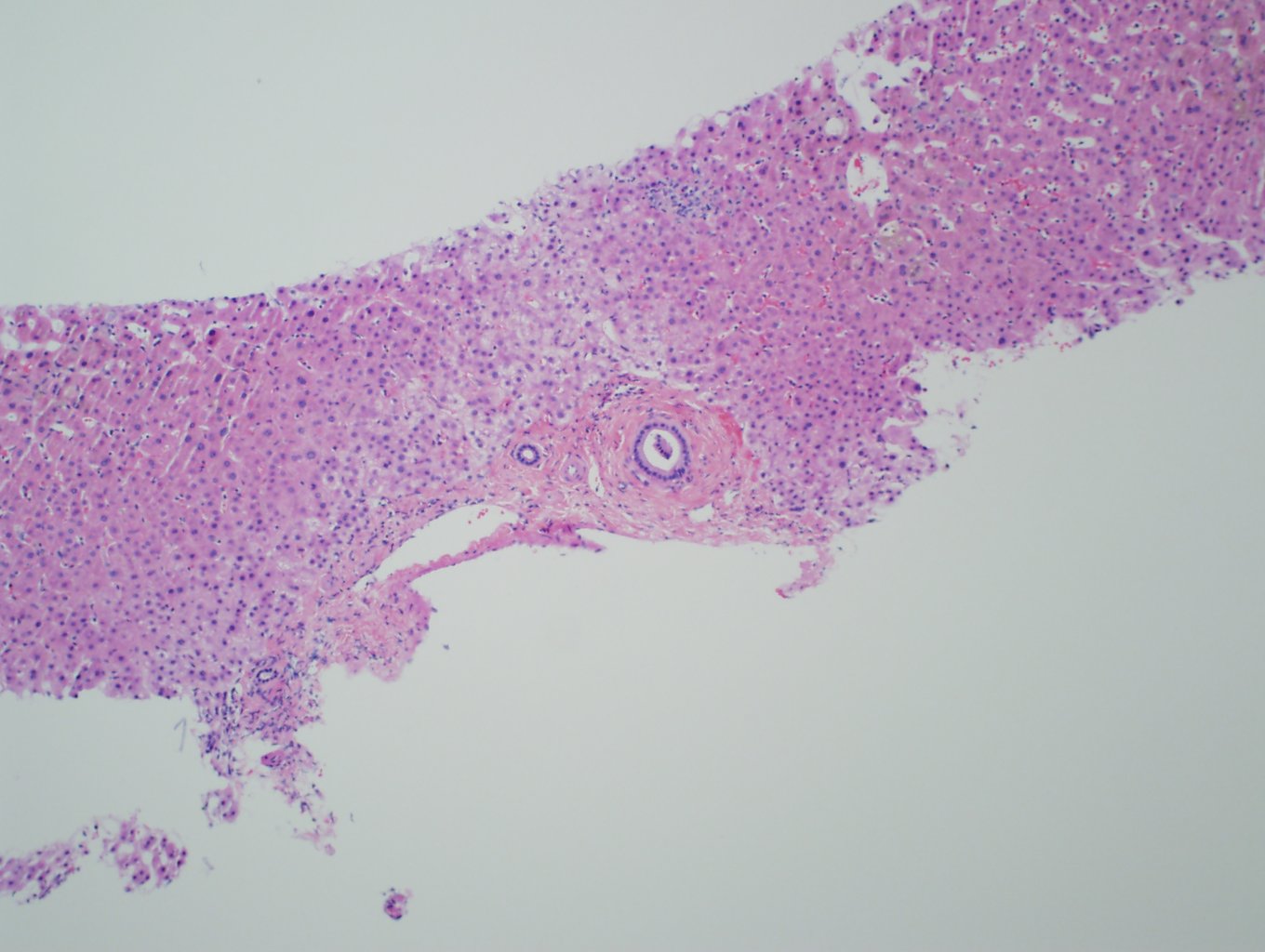
Figure 4: Normal bile ducts in portal tract.
Patient was treated with fluids, anti-histaminic, ursodeoxycholic acid. Patient was unable to tolerate coleveselam. Impression was drug induced hepatitis, lisinopril was discontinued and patient improved clinically and biochemically. Discharge labs two weeks after discontinuation of lisinopril showed AST 80 U/L, ALT 70 U/L, ALP 1045 U/L and GGT 1212 U/L; total bilirubin of 3.93 mg/dl with conjugated fraction 2.43mg/dl. Patient was discharged uneventfully with follow up in Hepatology clinic. Six months after discontinuation of lisinopril ALP was 199 U/L, GGT 168 U/L with AST 19 U/L, ALT 17 U/L, total bilirubin 0.9mg/dl and conjugated bilirubin 0.21mg/dl. Patient is currently asymptomatic and icterus has resolved.
Discussion:
ACE-I has been used widely for coronary artery disease, hypertension and diabetic nephropathy and approximately 159 million prescriptions for ACE-I are written annually. Recent JNCC guidelines recommended ACE-I to be used as first line anti-hypertensives for patients with CKD and diabetes. The common side effects known about ACE-I use are cough and angioedema, hypersensitivity. However not much awareness exists regarding ACE-I induced hepatotoxicity. It is important to consider ACE-I as an etiology for drug-induced liver injury (DILI) since continuation of the ACE-I beyond onset of hepatitis is fatal1.
Literature review shows multiple reports of DILI with captopril2, 3, ramipril4, fosinopril5, 6 and enalapril.2,7 Most commonly implicated ACE-I are enalapril and captopril. The usual presentation for ACE-I induced hepatotoxicity is cholestasis mediated hepatitis. Till date there have been four case reports published reporting lisinopril as cause of hepatitis 1, 8, 9 All 4 cases of lisinopril induced hepatotoxicity have shown a hepatocellular pattern of liver injury and did not show any cholestatic features. We report the first case of lisinopril induced cholestasis mediated hepatotoxicity.
In our case, patient had received enalapril for 5 months before initiation of lisinopril; however patient developed symptoms 2 years after initiation of lisinopril. The patient had no past medical history of liver or biliary tract disease. A thorough investigative workup was negative for autoimmune and other viral causes of hepatitis. Older case reports of lisinopril induced toxicity have shown similar histopathological findings of portal inflammation by lymphocytes without centrilobular zonal necrosis.9 There are various theories regarding possible mechanisms for DILI with lisinopril, namely terminal proline ring mediated bile stasis8, 10 and hypersensitivity to the sulfhydryl group.2 Discontinuation of metformin, pioglitazone, furosemide, atenolol and detemir did not result in clinical or biochemical improvement. Patient was initially continued on lisinopril since suspicion was low and then later discontinued. Similarity in histopathological findings along with a strong temporal relationship between lisinopril withdrawal and improved biochemical and clinical scenario, with absence of other constitutional symptoms and eosinophilia strongly point toward lisinopril-induced hepatotoxicity.
Our case had a long period of latency between drug intake and onset of hepatic injury which is consistent with other published reports of lisinopril induced hepatocellular injury9, 10, 11; however the mechanism responsible for latency or hepatotoxicity remains unclear. Earlier report postulate metabolic idiosyncratic reaction as a possible molecular mechanism for hepatocellular injury9. However our case is unique as the primary mode of injury appears to be cholestatic. Since our patient received enalapril before initiation of lisinopril without any adverse events, this case adds further controversy as to whether this patient could have been safely continued on other ACE-I except lisinopril or whether she would have developed hepatotoxicity if enalapril was continued. This case highlights further need for research to evaluate ACE-I induced hepatotoxicity. Currently the awareness for ACE-I induced liver injury is low and there are no guidelines guiding physician to monitor for possible hepatic adverse events. Further research is needed to delineate the mechanism by which ACE-I cause hepatotoxicity and to define possible risk factors.
Conclusion:
Discontinuation of ACE-I beyond recognition of DILI hepatitis usually leads to normalization of liver enzymes, however continuing or reinitiating ACE-I can be severe and potentially fatal. Thus, it is important to be aware of ACE-I as a possible cause of DILI, which can present with either hepatocellular or cholestatic mechanism and to promptly discontinue ACE inhibitor use. Currently there are no guidelines in place for monitoring of liver enzymes following initiation of ACE-I and more research is required to delineate possible mechanisms and prevent further DILI in such patients.
|
Acknowledgements None Competing Interests None Author Details GURPINDER SINGH, MD MBBS PGDHHM. Icahn School of Medicine at Mount Sinai Queens Hospital Center, 82-68 164 street, Jamaica, NY-11432, USA. AMIT KACHALIA, MD MBBS.Icahn School of Medicine at Mount Sinai Queens Hospital Center, 82-68 164 street, Jamaica, NY-11432, USA. JASPREET KAUR, MBBS. Icahn School of Medicine at Mount Sinai Queens Hospital Center, 82-68 164 street, Jamaica, NY-11432, USA. KINJAL KACHALIA, DNB MBBS.Icahn School of Medicine at Mount Sinai Queens Hospital Center, 82-68 164 street, Jamaica, NY-11432, USA. SHAOJUN LIU, MD. Icahn School of Medicine at Mount Sinai Queens Hospital Center,82-68 164 street, Jamaica, NY-11432, USA. VINCENT RIZZO, MD MBA, FACP. Icahn School of Medicine at Mount Sinai Queens Hospital Center,82-68 164 street,Jamaica, NY-11432, USA. CORRESPONDENCE: GURPINDER SINGH, Icahn School of Medicine at Mount Sinai Queens Hospital Center, 82-68 164 Street, Jamaica, NY 11432, USA. Email: drgurpinder@yahoo.co.in |
References
- Larrey D, Babany G, Bernuau J, et al. Fulminant hepatitis after lisinopril administration. Gastroenterology. 1990; 99:1832–3.
- Shionoiri H, Nomura S, Oda H, et al. Hepatitis associated with captopril and enalapril but not with delapril in a patient with congestive heart failure receiving chronic hemodialysis. CurrTher Res. 1987; 42:1171–6.
- Schattner A, Kozak N, Friedman J. Captopril-induced jaundice: report of 2 cases and a review of 13 additional reports in the literature. Am J Med Sci 2001; 322:236–240.
- Douros A, Kauffmann W, Bronder E, et al. Ramipril-induced liver injury: case report and review of the literature. Am J Hypertens. 2013 Sep;26(9):1070-5.
- Nunes AC, Amaro P, Mac AF, et al.Fosinopril-induced prolonged cholestatic jaundice and pruritus: first case report. Eur J GastroenterolHepatol 2001; 13:279–282.
- Chou JW, Yu CJ, Chuang PH, et al.Successful treatment of fosinopril-induced severe cholestatic jaundice with plasma exchange. Ann Pharmacother. 2008 Dec;42(12):1887-92.
- Da Silva GH, Alves AV, Duques P, et al. Acute hepatotoxicity caused by enalapril: a case report. J Gastrointestin Liver Dis. 2010 Jun;19(2):187-90.
- Hillburn RB, Bookstaver D, Whitlock WL. Angiotensin-converting enzyme inhibitor hepatotoxicity: further insights. Ann Pharmacother. 1993; 27:1142–3. Letter.
- Zalawadiya SK, Sethi S, Loe S, et al. Unique case of presumed lisinopril-induced hepatotoxicity. American Journal of Health-System Pharmacy August 15, 2010 vol. 67 no. 16 1354-1356.
- Hagley MT, Hulisz DT, Burns CM. Hepatotoxicity associated with ACE inhibitors. Ann Pharmacother. 1993; 27:228–31.
- Droste HT, de Vries RA. Chronic hepatitis caused by lisinopril. Neth J Med. 1995; 46:95–8.

The above article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.




