Chest pain and syncope in a middle-aged man
Deacon Zhao Jun Lee, Karan Saraf and Paul Sheridan
Cite this article as: BJMP 2014;7(4):a735
|
|
Abstract A 46 year old man presented to the Emergency Department with chest pain and collapse with loss of consciousness. The history, examination and investigation findings are detailed below followed by five questions surrounding the pathophysiology, diagnosis and management of the condition. Keywords: Brugada syndrome, channelopathy, sudden cardiac death, ventricular tachycardia, ventricular fibrillation, risk stratification, internal cardioverter defibrillator |
Case history
A 46 year-old man presented to the Emergency department with chest pain and collapse, associated with loss of consciousness lasting several minutes. He had no significant past medical history and he had no risk factors for coronary artery disease. However, he did note a similar episode of collapse and loss of consciousness one year prior for which he did not seek medical attention. There was no known family history of heart disease or sudden death.
On examination he was haemodynamically stable with a blood pressure of 130/80 mmHg and heart rate of 85 beats per minute. Jugular venous pressure was measured at 2cm above the sternal angle and heart sounds were normal with no added sounds. His oxygen saturation was 98% on air and chest was clear to auscultation. Chest X-Ray demonstrated clear lung fields and laboratory investigations, including electrolytes and cardiac troponin T were within normal limits. Echocardiography showed a structurally normal heart. Figure 1 shows his 12-lead electrocardiogram (ECG) on admission.
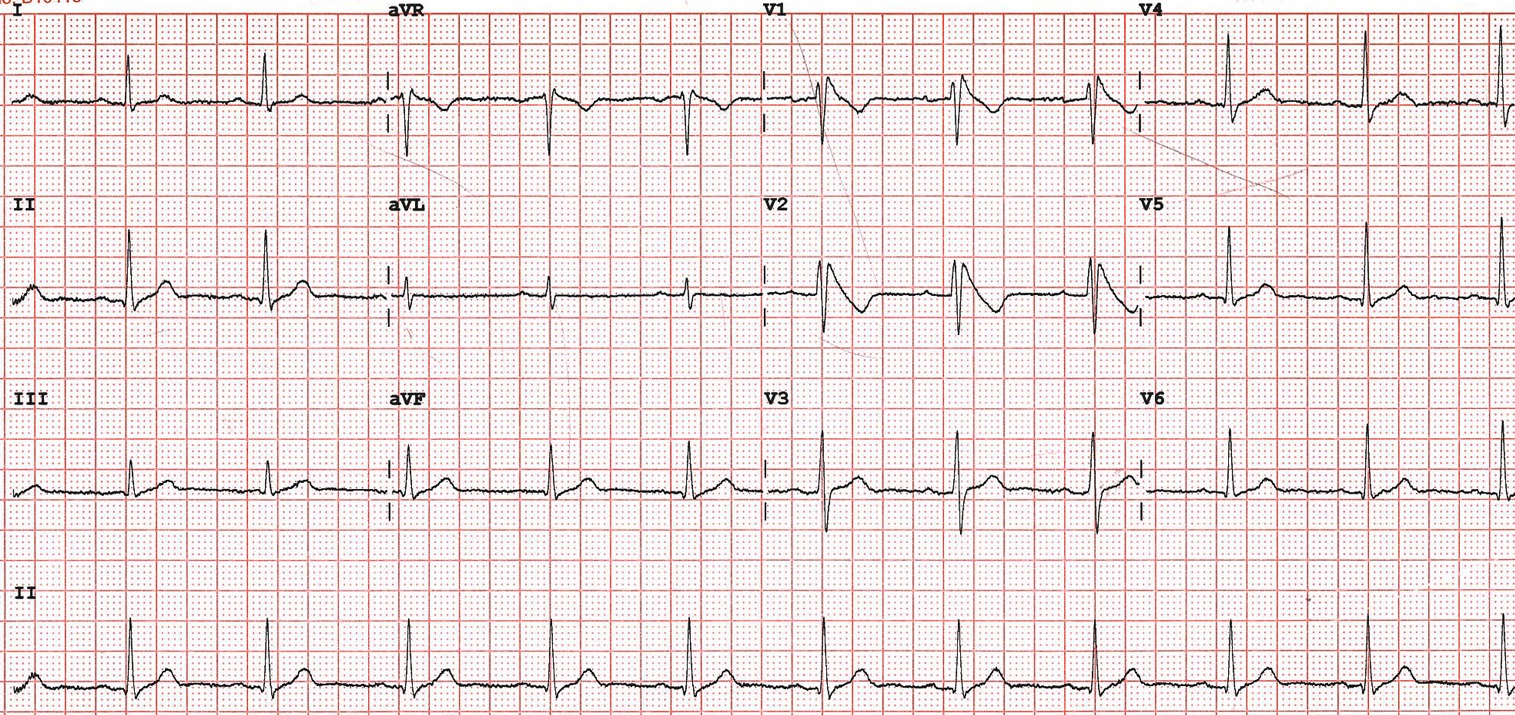
Figure 1 – 12 lead ECG on admission
Questions
1. What is the likely diagnosis based on the clinical presentation and ECG findings?
2. What life-threatening arrhythmias can arise from this condition?
3. What is the pathophysiology of this condition?
4. How is the diagnosis of this condition made?
5. What treatment options are available for patients with this condition?
Answers
1. What is the likely diagnosis based on the clinical presentation and ECG findings?
Short answer
Brugada syndrome
Long answer
The ECG shows coved Type 1 ST segment elevation in keeping with a diagnosis of Brugada syndrome.
First described in 1992, Brugada syndrome is a primary cardiac electrical disease or channelopathy that is accompanied a structurally normal heart and carries an association with sudden cardiac death1.
Presentation often occurs in the third or fourth decades of life, with a male preponderance of 8:1, however sudden cardiac death due to Brugada syndrome has also been seen in patients at the extremes of age. It is estimated that it accounts for up to 20% of all sudden cardiac deaths in patients without structural heart disease, ischaemia or electrolyte abnormalities2. It is most commonly seen in south-east Asia, especially Thailand, where its incidence is around 1%, and is much less common in western countries1.
2. What life-threatening arrhythmias can arise from this condition?
Short answer
Brugada syndrome is associated with increased risk of ventricular tachycardia (VT), often polymorphic, and ventricular fibrillation (VF).
Long answer
Brugada syndrome often manifests clinically in the form of syncope or sudden cardiac death. It is most commonly associated with polymorphic VT and VF, which may or may not terminate spontaneously. There are a large proportion of patients with Brugada syndrome who never experience any symptoms and indeed, may not ever even be identified as having the condition, unless they are found to have incidental ECG abnormalities as part of routine medical testing or are under investigation for another problem3.
3. What is the pathophysiology of this condition?
Short answer
Brugada syndrome is understood as a genetic cardiac channelopathy, a disorder produced by the dysfunction of a cardiac ion channel participating in the action potential which can result in electrical change favouring the development of arrhythmias. Inheritance of the condition occurs via an autosomal dominant mode of transmission with incomplete penetrance4.
Long answer
Brugada syndrome is a genetic disorder, with a loss-of-function mutation of the SCN5A gene implicated in about 30% of sufferers. This gene codes for the α-subunit of the cardiac sodium channel. Other mutations of sodium and calcium channels have also been found. In inherited cases, the gene is passed in an autosomal dominant fashion, though sporadic mutations are also seen.
There is increased susceptibility to ventricular arrhythmias, because of altered depolarisation within the right ventricle. In SCN5A mutations, the defect in sodium channels leads to decrease in the sodium current and a shortening of the cardiac action potential by blunting phase 0 depolarisation. Potassium channels are also affected, with an increased number of transient outward potassium channel currents. This imbalance in the myocytes between sodium and potassium concentrations means the overall effect is to shorten the refractory period, making the myocytes more prone to re-entrant circuits, leading to the development of VT and degeneration to VF2,5.
4. How is the diagnosis of this condition made?
Short answer
Brugada syndrome is characterised by electrocardiographic changes demonstrating coved ST segment elevation in the right precordial leads.
Long answer
Electrocardiographic abnormalities constitute the hallmark of Brugada syndrome. There are three different ECG patterns and in all three types, the ECG shows ≥2mm J point (junction between the termination of QRS complex and beginning of ST segment) elevation and a characteristically shaped ST segment in the right precordial leads6.
Type I has a ‘coved’ pattern ST segment elevation ≥2mm, with a descending terminal portion in at least one right precordial lead.
Type II has a ‘saddle-back’ ST segment elevation ≥1mm and has a high elevation in its initial portion.
Type III has either coved or saddleback ST elevation but is less accentuated than types I or II (<1mm).
Although all the 3 patterns can be present in patients with Brugada syndrome, only the presence of a type-1 ECG pattern defines the diagnosis of the condition2,7. The patterns for type II or III are not diagnostic, and carrying out a Class I anti-arrythmic drug (AAD) test to confirm the diagnosis is recommended. This can be done with AADs such as ajmaline, flecainide or procainamide, though currently ajmaline is preferred due to its higher sensitivity in revealing Brugada type ECG changes1.
It is worth noting that the resting ECG changes associated with Brugada syndrome (in particular type I) are often transient, and therefore, in someone in whom the diagnosis is suspected, an AAD test may be indicated even if there are no resting spontaneous ECG abnormalities evident6.
Differential diagnoses of Brugada syndrome must be approached with care as ST segment elevation is associated with a wide variety of benign and malignant pathophysiologic conditions3.
5. What treatment options are available for patients with this condition?
Short answer
Currently, the implantable cardioverter defibrillator is the only proven effective treatment in the prevention of sudden cardiac death.
Long answer
Management of Brugada syndrome is focused on risk stratification of patients to prevent arrhythmic death in high risk individuals. ICD implantation can prevent sudden cardiac death in these groups1. Newer devices are now also being used, including the subcutaneous ICD which is implanted in a subcutaneous pocket and does not require any endovascular leads in the heart or access to the central venous circulation8.
Pharmacological options are focused on rebalancing the ion channel current active during the early phases of the epicardial action potential in the right ventricle3,9. Some studies have evaluated the role of quinidine in the treatment of Brugada syndrome and found it to be effective in preventing polymorphic VT and VF in this condition10. Quinidine has also been proposed as an alternative to ICD implantation in children and infants too young to receive an ICD11,12.
Data relative to the use of cryosurgical treatments or ablation therapy in Brugada syndrome are very limited at this point in time3.
Patient outcome
The patient underwent successful implantation of a subcutaneous cardioverter defibrillator. Figure 2 and Figure 3 show chest radiographs of the leadless device.
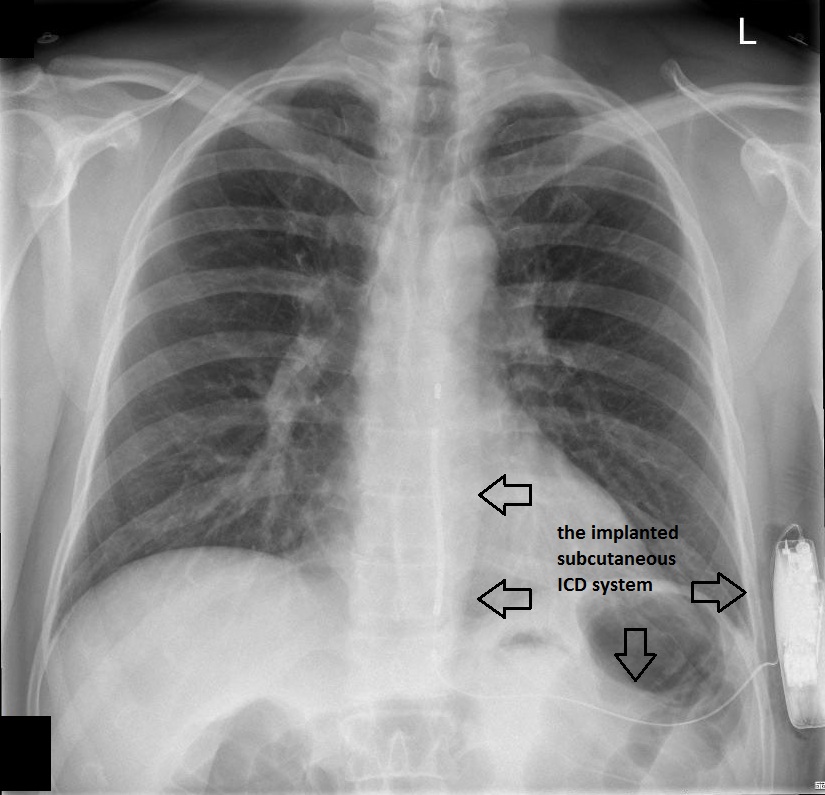
Figure 2 – Chest radiograph (PA view) showing the implanted subcutaneous ICD
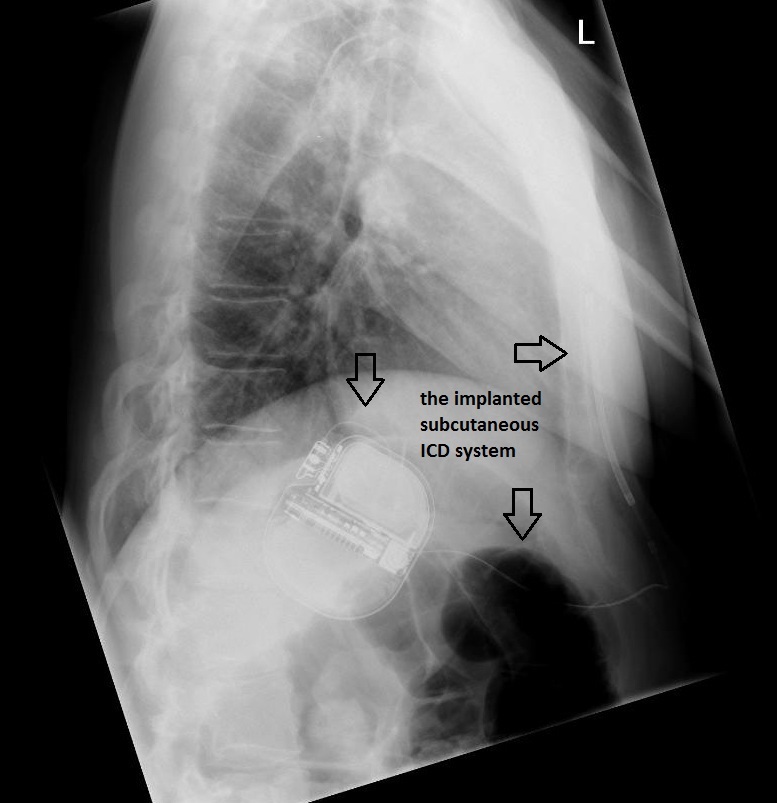
Figure 3 – Chest radiograph (lateral view) showing the implanted subcutaneous ICD
|
Competing Interests None declared Author Details DEACON ZHAO JUN LEE, MBCHB MRCP, Sheffield Teaching Hospitals, UK. KARAN SARAF, MBCHB, Sheffield Teaching Hospitals, UK. PAUL SHERIDAN, MBCHB MRCP PhD, Sheffield Teaching Hospitals, UK. CORRESPONDENCE: DEACON ZHAO JUN LEE, Sheffield Teaching Hospitals, Northern General Hospital, Herries Road, Sheffield, S5 7AU. Email: deacon.lee.04@aberdeen.ac.uk |
References
- Boussy T, Sarkozy A, Chierchia GB et al. The Brugada Syndrome: Facts and Controversies. Herz 2007;32:192-200
- Antzelevitch C, Brugada P, Borggrefe M et al. Brugada syndrome: report of the second consensus conference: endorsed by the Heart Rhythm Society and the European Heart Rhythm Association. Circulation 2005;111:659-70
- Antzelevitch C. Brugada Syndrome. PACE 2006;29:1130-59
- Benito B, Brugada R, Brugada J et al. Brugada Syndrome. Progress in Cardiovascular Diseases 2006;51(1):1-22
- Herbert E, Chahine M. Clinical aspects and physiopathology of Brugada Syndrome: a review of current concepts. Can J Physiol Pharmacol 2006;84:795-802
- Richter S, Sarkozy A, Chierchia GB et al. Variability of the diagnostic coved-type ECG during long-term follow-up of patients with Brugada syndrome and primary prophylactic ICD implantation. Eur Heart J 2006,27:Suppl 1:AB88662
- Wilde AAM, Antzelevitch C, Borggrefe M et al. Proposed diagnostic criteria for the Brugada syndrome. Eur Heart J 2002;23:1648-1654
- Weiss R, Knight BP, Gold MR et al. Safety and efficacy of a totally subcutaneous implantable-cardioverter defibrillator. Circulation 2013;128:944-953
- Márquez MF, Salica G, Hermosillo AG et al. Ionic basis of pharmacological therapy in Brugada syndrome. J Cardiovasc Electrophysiol. Feb 2007;18(2):234-40
- Belhassen B, Glick A, Viskin S. Efficacy of quinidine in high-risk patients with Brugada syndrome. Circulation 2004;110(13):1731–7
- Probst V, Evain S, Gournay V et al. Monomorphic ventricular tachycardia due to Brugada syndrome successfully treated by hydroquinidine therapy in a 3 year old child. J Cardiovasc Electrophyiol 2006;17:97-100
- Probst V, Denjoy I, Meregalli PG et al: Clinical aspects and prognosis of Brugada syndrome in children. Circulation 2007;115:2042-2048

The above article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.




