Case Report: Co-Existing Sinus and Atrio-Ventricular Node Dysfunction Following Stress-Induced Cardiomyopathy
Raja Ezman Faridz Raja Shariff, Lim Chiao Wen, Rizmy Najme Khir, Khairul Shafiq Ibrahim, Ahmad Bakthiar Ahmad Radzi & Sazzli Kasim
Cite this article as: BJMP 2019;12(1):a002
|
|
Keywords: Takotsubo Cardiomyopathy, Stress-Induced Cardiomyopathy Abbreviations: SCM: Stress-Induced Cardiomyopathy; ECG: electrocardiogram; SND: Sinus Node Dysfunction; AVN: Atrioventricular Node |
Background
Stress-Induced Cardiomyopathy (SCM), also known as Takotsubo Cardiomyopathy or Apical Balooning Syndrome, is an acute, transient and non-ischaemic cause of left ventricular dysfunction often precipitated by periods of stress1. Diagnosis often follows evidence of left ventricular hypokinesia despite a normal coronary angiography. Prevalence is often underestimated, with an estimated 7% of suspected myocardial infarctions being in fact SCM2. We report a unique case of multi-nodal dysfunction following SCM.
Case Report
A 73-year old lady presented to our emergency department following a sudden onset of central, non-radiating chest heaviness 8 hours prior. She was a known chronic smoker of 20 pack years, and hypertension which had been left untreated for over 10 years. An initial electrocardiogram (ECG) revealed sinus bradycardia and T-wave inversions in the inferior, septal and lateral leads (Figure 1). Her Troponin-I levels was raised at 6532 pg/ml. She was treated as a Non-ST elevation myocardial infarction and was admitted to the coronary care unit for closer monitoring. She was kept on telemetry overnight, which disclosed several episodes of bradycardia. Rhythm strip revealed various transient conduction defects, including that of sinus node dysfunction (SND) and atrioventricular node (AVN) dissociation, although she remained asymptomatic throughout (Figure 2).
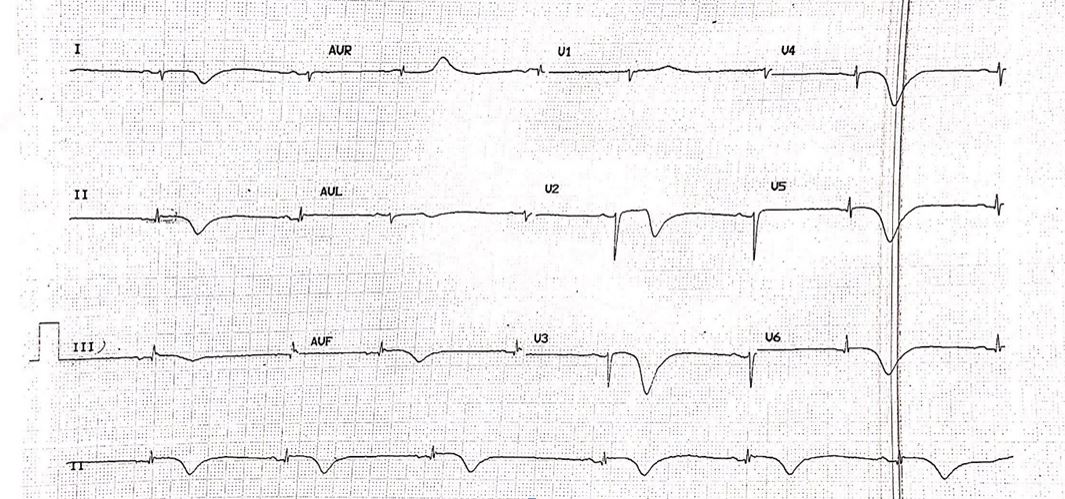
Figure 1: Electrocardiogram revealing sinus bradycardia, with T-wave inversion in the inferior, septal and lateral leads.
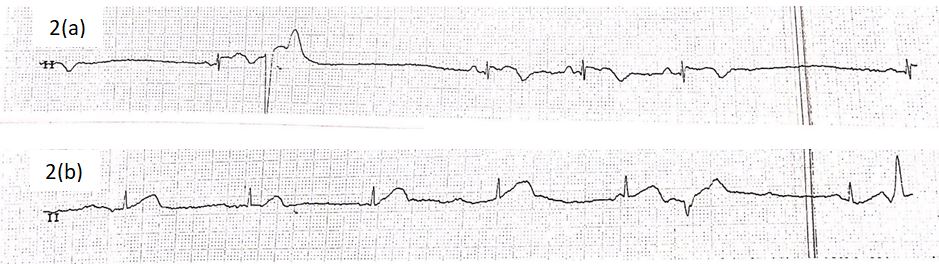
Figure 2: Telemetry rhythm strip revealing transient episodes of (a) sinus node dysfunction (SND) and (b) atrioventricular node (AVN) dissociation.
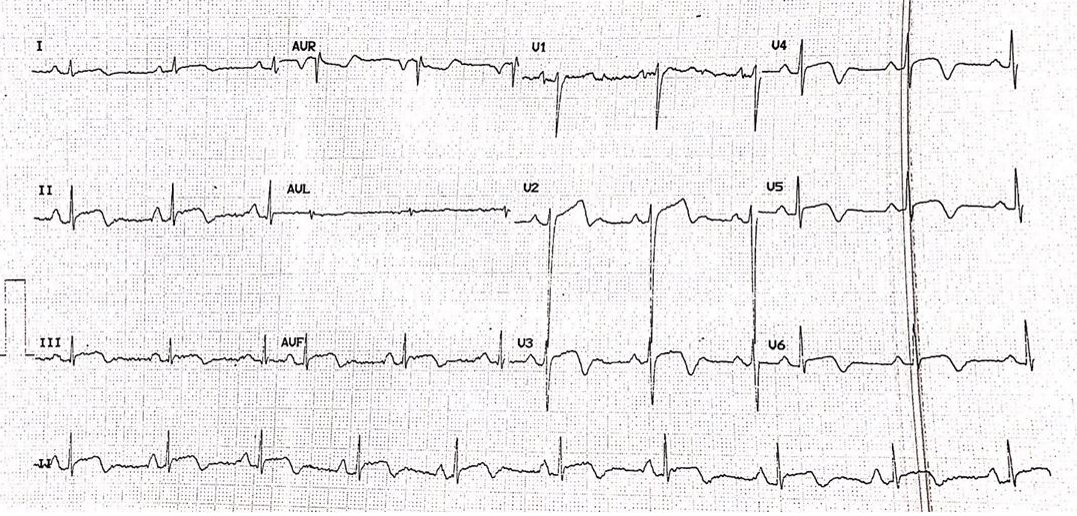
Figure 3: Electrocardiogram revealed ST-segment elevation with associated T-wave inversions in the inferior, septal and lateral leads.
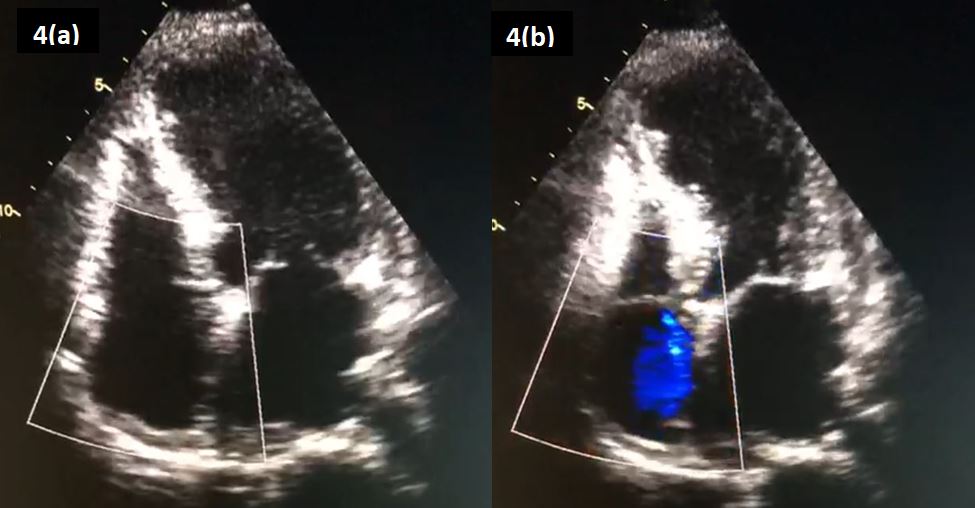
Figure 4: An ‘Apical 4-Chamber’ view on echocardiography, revealing an akinetic apex on (a) diastole and (b) systole.
Unfortunately, following an episode of chest pain the following morning, her troponin levels and an electrocardiogram were repeated, showing a rise of the former to 12 996 pg/ml. A repeat ECG revealed evidence of ST-segment elevation in previously affected leads (Figure 3). She was brought into the catheterization laboratory within 1 hour. Her coronary angiogram showed no evidence of coronary obstruction. An echocardiogram was performed, which revealed an akinetic apex (Figure 4).
Upon further history taking, it was revealed that she was recently made redundant from her job as a cleaner, several hours prior to her presentation to the emergency department. Prior to that, she denied any other emotional or physical stressors. She was diagnosed as having Stress-Induced Cardiomyopathy (SCM). Following an observational period of close to 48 hours, she was allowed home. A 48-hour Holter monitoring was performed approximately 3 weeks from her initial admission, which returned unremarkable. A repeat echocardiography was also performed, revealing normal wall motion abnormality which further support a transient SCM.
Discussion
Despite being transient, multiple complications can arise from the condition, including arrhythmias. Prevalence of arrhythmias varies greatly, depending on population and types of defect (15% suffering atrial fibrillation, 2-5% of tachyarrhythmias, 2-5% of bradyarrhythmias and 5% of AVN dissociation amongst others)3,4. This is largely due to evidence being based on retrospective case report and series, leading to severe underestimation of their true prevalence. We suspect cases of sinus node dysfunction are far more uncommon, with only a handful of case report of note, and one retrospective review of 816 patients quoting a rate of 1.3%5. There are no reports of concomitant sinus node and atrio-ventricular node dysfunction, to our knowledge.
Proposed mechanisms for SCM-induced nodal dysfunction include reduced coronary flow to conduction tissues due to left ventricular dyskinesia, cathecolamine-driven coronary and microvascular vasospasm leading to both reduced blood supply and direct cardio-toxicity effects, and continual ischaemia-driven fibrosis of nodal tissue6. However, there have been reports of SND-triggered SCM, likely secondary to adrenergic compensative activation following bradycardia events. In both scenarios, pre-existing, subclinical SND may lower the threshold of developing significant, symptomatic bradycardia7-10. This is important to note, as majority of patients affected by SCM are post-menopausal women whom are already at risk of age-related SND.
In our patient, the SCM may have likely induced both SND and AVN dissociation, as subsequent 48 hours Holter monitoring, 3 weeks from presentation, was unremarkable. Furthermore, the patient denies any previous syncopal or pre-syncopal symptoms. However, the possibility of subclinical SND could still have existed, as we had earlier discussed, and ideally an internal loop recorder for prolonged monitoring, catheter-based electrophysiology studies and a Cardiac Magnetic Resonance Imaging to detect nodal and conduction tissue fibrosis would assist in ruling out pre-existing nodal dysfunction. However, due to financial and pragmatic reasons (as patient was asymptomatic), the patient declined further investigations, opting for periodic clinic reviews instead.
Conclusion
Both nodal and conduction tissue blocks are a rare but significant complications that can occur following SCM. The occurrence of SND following SCM should lead clinicians to routinely investigate for pre-existing conduction tissue diseases, if not already performed and allows for earlier device implantation if deemed indicated.
|
Competing Interests None declared Author Details RAJA EZMAN FARIDZ RAJA SHARIFF (MBHCHB, MRCP) - CORRESPONDING AUTHOR 1 Universiti Teknologi Mara, Sungai Buloh, Malaysia. LIM CHIAO WEN (MBBCH, MRCP) 1 Universiti Teknologi Mara, Sungai Buloh, Malaysia. RIZMY NAJME KHIR (MBBCH, MRCP) 1 Universiti Teknologi Mara, Sungai Buloh, Malaysia. KHAIRUL SHAFIQ IBRAHIM (MBCHB, MRCP) 1 Universiti Teknologi Mara, Sungai Buloh, Malaysia. AHMAD BAKTHIAR AHMAD RADZI (MBBS, MMED) 1 Universiti Teknologi Mara, Sungai Buloh, Malaysia. SAZZLI KASIM (MBBCh, BAO, BMedSc (NUI, Cork), MRCPI, CSCST (Ire), FNHAM) 1 Universiti Teknologi Mara, Sungai Buloh, Malaysia. CORRESPONDENCE: RAJA EZMAN FARIDZ RAJA SHARIFF, Universiti Teknologi Mara Sungai Buloh, Jalan Hospital, 47000, Sungai Buloh, Selangor, Malaysia. Email: rajaezman@gmail.com |
References
- Kasim S, Moran D, O'Donnabhain R, et al. Takotsubo Cardiomyopathy – A Large Cohort, Multi-Centre Follow Up. Irish Jounral of Medical Science 2010; 179:S409.
- Dawson DK. Acute Stress-Induced (Takotsubo) Cardiomyopathy. Heart 2017; 0:1-7.
- Gamble DT, Shuttleworth KJ, Scally C, et al. Takotsubo cardiomyopathy with severe bradyarrhythmia following epidural insertion. BMJ Case Rep 2016; 0:1-3.
- Templin C, Ghadri JR, Diekmann J, et al. Clinical Features and Outcomes of Takotsubo (Stress) Cardiomyopathy. N Engl J Med 2015; 373:929–938.
- Yassin AS, Subahi A, Abubakar H, et al. Sick Sinus Syndrome and Takotsubo Cardiomyopathy. Case Reports in Cardiology 2018; 0:1-5.
- Limm BN, Hoo AC & Azuma SS. Variable Conduction System Disorders in Takotsubo Cardiomyopathy: A Case Series. Hawaii Journal of Medicine & Public Health 2014; 73(5):148-151.
- Brunetti ND, Ieva R, Correale M, et al. Combined exogenous and endogenous catecholamine release associated with Tako-Tsubo like syndrome in a patient with atrio-ventricular block undergoing pace-maker implantation. Acute Cardiac Care 2011; 13(2):112-114.
- Ueyama T, Kasamatsu K, Hano T, et al. Emotional stress induces transient left ventricular hypocontraction in the rat via activation of cardiac adrenoceptors: a possible animal model of ‘tako-tsubo’ cardiomyopathy. Circulation Journal 2002; 66(7):712-713.
- Ueyama T, Senba E, Kasamatsu K, et al. Molecular mechanism of emotional stressinduced and catecholamine-induced heart attack. Journal of Cardiovascular Pharmacology 2003; 41:S115-S118.
- Syed FF, Asirvatham SJ, & Francis J. Arrhythmia occurrence with takotsubo cardiomyopathy: a literature review. Europace 2011; 13(6):780-788

The above article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.




