Blocked percutaneous endoscopic gastrostomy tube - an unusual cause
Vijay Joshi and Ashis Banerjee
Cite this article as: BJMP 2009:2(2) 46-47
|
Case report
An 82 year old lady, who had suffered multiple strokes in the past and was currently on long term percutaneous endoscopic gastrostomy( PEG) feeding, was admitted as an emergency from a nursing home with a two week history of productive cough and fever. She had been on PEG feeding since her first stroke six years previously. The first PEG tube (placed in 2001) subsequently fell out of position, and a second tube (15 French Frecka PEG tube) was inserted in 2003.
On admission, she was pyrexial, dehydrated, and hypoxic on room air. Chest examination revealed bilateral crackles and neurological examination revealed expressive dysphasia, and spastic weakness in both lower limbs. Abdominal examination revealed an inflamed PEG site with purulent discharge. Blood tests revealed raised inflammatory markers with neutrophilia (WBC 20 x 109 /L with a neutrophil count of 12 x 109/L) and a raised C-reactive protein at 193 mg/L.
She was managed with intravenous fluids and antimicrobial therapy (tazocin and metronidazole) for possible aspiration pneumonia. Vancomycin was subsequently commenced as methicillin resistant staphylococcus aureus (MRSA) was isolated from the PEG site. As she remained stable, PEG feeding was recommenced.
A week following her admission she became unwell with an episode of vomiting and choking following PEG feeding. This was associated with difficulty in infusing feeds and medications through the PEG tube. Multiple flushes through the tube were unsuccessful. The tube was found to be persistently blocked and lacked free mobility within the tract.
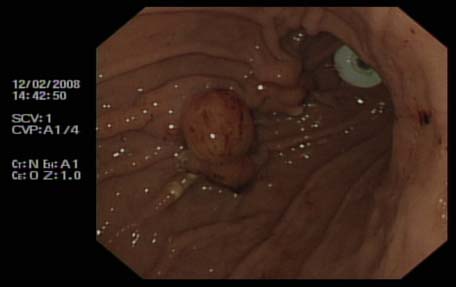
Urgent upper gastro intestinal endoscopy revealed a buried bumper as the cause of blockage of the PEG tube. This necessitated insertion of a new PEG tube (9 French Frecka) for enteral feeding. The old PEG tube was removed surgically under local anaesthesia in due course. As the removal of the buried bumper was found to be very difficult endoscopically, and surgical intervention was deemed to be inadvisable in view of co morbidities, the bumper was left in situ. Feeding was recommenced through a new tube. In view of persistent discharge through the PEG site, abdominal ultrasound examination was performed, revealing a possible gastro-cutaneous fistula. No local collection was seen around the PEG wound.
As the patient remained clinically stable, she was discharged home with necessary instructions to her carers for regular flushing of the PEG tube with water, before and after each feed, to prevent further blockages.
Fig 1: Buried bumper (stomach-lower body)
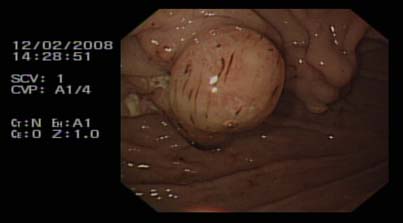
Fig 2: New peg and buried bumper
Discussion
PEG is primarily used for long term (longer than 6 weeks) enteral alimentation for patients with impaired swallowing (e.g. from stroke, degenerative neurological disease, head injury, and oropharyngeal malignancy). However numerous complications have been reported since its introduction in 1980.
Buried bumper syndrome (BBS) is an uncommon but well documented complication of PEG insertion, first described in 1988 1. It is usually a late complication occurring up to 3 years post PEG insertion and reported to occur in 0.3-2.4 % of patients2.
The internal bumper of the PEG tube should normally sit snugly against the anterior gastric wall, and this is confirmed endoscopically at the time of initial placement. BBS develops when there is migration of the internal bumper/flange through or into the anterior abdominal wall. This probably occurs as a result of excessive tension between the internal and external bumpers, from over-tightening of the external flange, leading to gastric wall erosion. During migration it becomes lodged along the gastrostomy tube tract between the gastric and abdominal walls. Once epithelialisation occurs the bumper gets covered with gastric mucosa3.
The diagnosis of BBS should be suspected if localised abdominal pain, peri-tubal leakage or inability to infuse feed occurs. Initial measures to deal with a blocked tube include flushing with warmed water, and occasionally passage of a flexible wire through the lumen, in order to unblock any obstruction. Tube obstruction is usually related to the administration of protein-enriched formulae or medications, especially if the tube size is 9 French. Fungal colonisation may also lead to tube blockage, requiring specific solutions for flushing the tube4. Tube exchange should only be considered if the gastrocutaneous tract is mature (6 weeks or longer after placement of the tube).
Endoscopy is confirmatory in cases of BBS. The internal bumper is not seen, and the site of the PEG is indicated by an elevated area of submucosa with a central depression. Failure to recognise BBS can result in gastric perforation and gastrointestinal haemorrhage or intra abdominal sepsis, peritonitis and even death5.
Ideally, the buried bumper should be removed even if the patient is asymptomatic, to avoid potential complications from continued tube migration until it is completely impacted in the abdominal wall. The literature describes various methods of dealing with this complication. Endoscopic ultrasound of the gastric wall with a catheter US probe can facilitate the localisation of the bumper and also provides information regarding feasibility of surgical or endoscopic removal of PEG tube6.
Regular and optimal PEG care has been vital in identifying and prevention of this complication. During daily cleaning of the external PEG site, the PEG should be pushed in approximately 1 cm and rotated prior to repositioning of the external bumper. The length of the tube outside the abdominal wall should be examined at regular intervals so that migration can be recognised5.
This report reinforces the fact that physicians should be aware of this recognised risk of PEG feeding and prompt referral for endoscopy is necessary to avoid serious consequences including gastro-intestinal bleeding, peritonitis and death. Similarly specific instructions should be given to carers for prevention of BBS.
| COMPETING INTERESTS None Declared AUTHOR DETAILS VIJAY JOSHI, Trust registrar in integrated medicine, Chase Farm Hospital, Enfield, UK ASHIS BANERJEE, Consultant in emergency medicine, Chase Farm Hospital, Enfield, UK CORRESPONDENCE: MR ASHIS BANERJEE, Consultant/honorary senior lecturer in emergency medicine, Chase Farm Hospital, The Ridgeway, Enfield EN2 8JL United Kingdom E-mail: libra19542003@yahoo.co.uk |
References
- Shallman RW, Norfleet RG, Hardache JM. Percutaneous endoscopic gastrostomy feeding tube migration and impaction in the abdominal wall. Gastrointest Endosc 1988; 34: 367–68.
- Venu RP, Brown RD. Pastika BJ Erickson LW. The buried bumper syndrome: a simple management approach in two patients. Gastrointest Endosc 2002; 56: 582–84.
- Anagnostopoulos GK, Kostopoulos P, Arvanitidis DM. Buried Bumper Syndrome with a fatal outcome, presenting early as gastrointestinal bleeding after percutaneous endoscopic gastrostomy placement. J Postgrad Med 2003; 49:325–27
- Iber, FL, Lusak, A, Patel, M Importance of fungus colonization in failure of silicone rubber percutaneous gastrostomy tubes (PEGs) Dig Dis Sci, 1996, 41: 226-231
- Braden B, Brandstaetter M, Caspary WF, Seifert H. Buried bumper syndrome: treatment guided by catheter probe US. Gastrointest Endosc 2003;57:747-51
- Ma MM, Semalacher EA, Fedorak RN, Llor EA, Duerksen DR et al The buried gastrostomy bumper syndrome: prevention and endoscopic approaches to removal. Gastrointest Endosc 1995 ; 41:505-8

The above article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.




