The Association of NCF1 Gene with the Severity of Malaria
Shakirullah, Muhammad Arshad, Sohail Afzal, Bahadar Zaib and Saba Haq
Cite this article as: BJMP 2011;4(2):a416
|
|
Abstract The phagocyte oxidase 47 (P47Phox) is produced by the NCF1 gene which is located on chromosome 7. The P47 Phox forms an important component of the NADPH oxidase complex enzyme which leads to the production of reactive oxygen species (ROS). The NCF1 gene exists in two versions, one is a wild type gene with GTGT at the start of exon 2. The other is a pseudogene and it has its GT deletion at the start of exon2 which leads to passive production of ROS. The role of ROS in malaria was studied through the restriction enzymeGeobacillus stearothermophilus GR75 (BsrG1) found in the NCF1 gene. This enzyme digests only the pseudogene and has no impact on the wild type gene. The comparison of 88 malarial patients with 100 healthy individuals proves that there was no association of NCF1 gene with malaria because the P value was greater than 0.05. Background: Malaria is a mosquito-borne infectious disease which is caused by a eukaryotic protist of the genus Plasmodium. The most fatal form of the disease is caused by Plasmodium falciparum. The neutrophil cytosolic factor 1 (NCF1), also known as p47 phox, is an important subunit of NADPH oxidase which plays a role in the production of ROS. The forming of ROS is a pivotal component of innate immunity against parasitic and bacterial infection. Aim: The aim of study was to find out that whether the NCF1 gene and ROS had a role in malaria. Method: Samples from 88 malarial patients and 100 healthy individuals were processed with the restriction enzyme BsrGI. Conclusion: It was found that the NCF1 gene and ROS have no association with malaria. |
Introduction
Malaria is a major worldwide scourge, infecting and killing several million individuals each year1. Malaria is common in mostly tropical and subtropical areas such as The Americas, Asia and Africa2, 3.
The NADPH oxidase complex is responsible for the reduction of oxygen in cells, yielding a superoxide anion (O2˚-) that is subsequently converted into other ROS; including hydrogen peroxide (H2O2) and the hydroxyl radical (OH˚)4.
The Sequence analysis showed that the NCF1 wild type gene is 15,236 bp long, contains 11 exons and has an intron/exon structure identical to the highly homologous pseudogene5. The pseudogene which is highly homologous to the wild type gene is located on the same region of the chromosome, which is 7q11.23 of chromosome 76. Comparative sequence analysis between the wild type gene and pseudogene demonstrates greater than 98% homology but the pseudogene has a GT deletion (ΔGT) at the start of exon 27. The genomic pattern of wild type NCF1 gene and its pseudogene may influence the production of reactive oxygen species (ROS) in parasitic and bacterial infections and also in autoimmune diseases.
During malarial infection, the ROS production can contribute to rapid parasite clearance in mild malaria8 but in severe malaria the high capacity production of ROS was associated with anaemia. This means that ROS has a possible role in both parasite clearance and anaemia during P.falciparum infection9. Genetic variation in components of the leukocyte NADPH oxidase may, therefore, influence disease susceptibility to, and disease duration of parasitic infection and autoimmune disease10.
Study Design
Inclusion and exclusion criteria
Patients who had fever with malarial parasites detected microscopically from blood smears and had no evidence of other illnesses were selected. Patients were excluded if they developed other illnesses within three days of admission or if there was any other present infection.
Relatives of patients in the hospital and in the laboratory and members of the community without malaria or any other febrile illness were included after clinical evaluation. These formed the control group of healthy individuals.
Patients and healthy individuals
To determine the association of NCF1 gene in malaria, the blood samples were collected from malarial patients and healthy individuals in storage tubes coated with EDTA. Malaria was diagnosed on the basis of clinical observation and positive smear test containing various types of plasmodium.
Materials and Methods
The restriction fragment length polymorphism (RFLP) method was performed to determine the prevalence of NCF1 gene GT deletion (ΔGT) among patients with malaria and among healthy individuals in a Pakistani population. In order to determine whether there was an association between NCF1 gene GT deletion (ΔGT) at the start of exon 2 with susceptibility to malaria, 88 malarial patients and 100 healthy individuals were genotyped for the GT deletion by restriction enzyme analysis.
Genetic Analysis
Genomic DNA of patients and of the healthy control subjects was extracted from venous blood samples using the nucleospin blood extraction kit (NucleoSpin® Blood, Germany) according to the manufacturer’s protocol. The NCF1 gene was analyzed by the restriction fragment length polymorphism (RFLP) method. The exon 2 was amplified using both forward and reverse primer as shown in Table 1. The reaction mixture (50 μl) for PCR was prepared in 0.2 ml tubes (Axygen®, California, USA) by adding the following: 1.2 μl of sample DNA (50 ng /μl), 5 μl (10X) from the PCR buffer (Fermentas, Burlington, Canada), 4 μl of 25mM magnesium chloride (MgCl2) (MBI Fermentas, Burlington, Canada), 3 μl of 2 mM deoxyribonucleotide triphosphates (dNTPs) mixture (MBI Fermentas, Burlington, Canada), 2.6μl of each forward primer (10 pm/μl), 2.6 μl of the reverse primers (10 pm/μl) and 1.2μl Taq DNA polymerase (MBI Fermentas, Burlington, Canada) in 30.40 μl nuclease free water with cycling conditions 95 °C for 5 min, followed by 35 cycles at 95 °C for 1 min, 60.6 °C for 1 min, 72 °C for 1 min and finally a 10 min extension at 72 °C.
The amplified products were then treated with the restriction enzyme BsrGI (Geobacillus stearothermophilus GR75) (Fermentas life science). For a 20 μl mixture we took 10 μl of PCR product, 2 μl 10X buffer tango, 1 μl BsrGI enzyme and 7 μl of nuclease free water to make the mixture volume up to 20 μl and checked the result on 2% agarose gel.
Table 1: sequence of primers and product size
| Deletion Exon | primers | sequence | Product size |
| DNCF2F | 5'-GCTTCCTCCAGTGGGTAGTG-3' | ||
| DNCF2R | 5-GCAAGACCCTGGGTGACAGA-3' | ||
| GTGT | 358 bp | ||
| GT | 356 bp |
Statistical analysis was performed using the Study Result Software Version 1.0.4 (CreoStat HB Frolunda, Sweden). The association of both types of genes in malaria patients and healthy individuals were compared using the χ2 or Fischer’s exact test. Similarly to elucidate whether there was an association between the age and gender of both patients and healthy controls, analysis was done using the T-test and chi-square test by the online Graphpade software.
Results
Characteristics of patients
The characteristics of patients and healthy individuals are mentioned in Table 2 and table 3.
Table 2: Total number of patients and healthy individuals with their respective mean ages
| Characteristic | Patients | Mean age | Control | Mean age | P value | ± SD |
| Total number of subjects | 88 | 22 | 100 | 26 | 0.43761 | 0.8627 |
| Adults > 22 year | 60 | 60 | ||||
| Children <22 year | 28 | 40 |
Table 3: Total number of Patients and healthy individuals enlisted with respective mean age.
| Characteristic | Patients | Mean age for patients | Control | Mean age for controls |
| No of males | 75 | 24 | 80 | 26 |
| No of females | 13 | 20 | 20 | 27 |
| Total | 88 | 22 | 100 | 26 |
A chi-square test was performed to test the null hypothesis regarding whether there was an association between gender and the number of subjects in the control group and patient groups. No statistically significant association was found, c2 (1, N = 188) = 0.559, p = 0.4545”. Similarly, a chi-square test was performed to test whether there was any association between the gender and ages of subjects in the control group and in the patient group. Again no statistically significant association was found, c2 (1, N = 188) = 0.299, p = 0.5848”.
PCR amplification of exon 2 of NCF1 gene
The exon 2 was amplified by polymerase chain reaction to obtain 358 base pair long regions in the case of the wild type gene and 356 base pair long regions in the case of the pseudogene. The products were treated with a restriction enzyme, Geobacillus stearothermophilus, GR75 (BsrGI) which digests only the wild type gene (GTGT), which meant two bands of 265 bp and 93 bp were obtained respectively. Whereas with the pseudogene (ΔGT) there was no digestion and the original band of 356 bp was obtained. When the individuals had both the wild type gene (GTGT) and the pseudogene (ΔGT)three bands of 265 bp, 93 bp and 356 bp were obtained, two of the wild type gene and one of the pseudogene respectively, which is shown in figure 1.
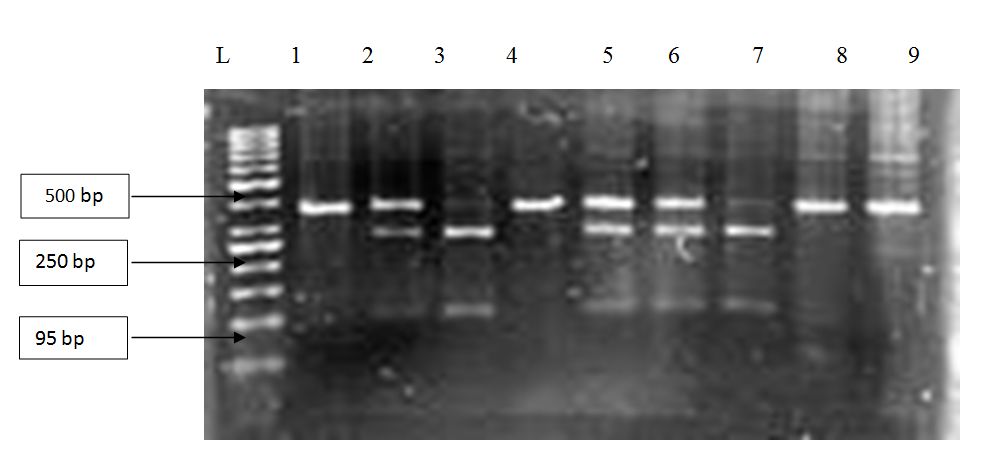
Figure 1: Agarose gel (2%) showing genotypes of eight patients with malaria. The GT deletions (ΔGT) were checked using the RFLP method, using the restriction enzyme BsrGI. The lane L contains 50 base pair markers, while lane 1 contains a negative sample and 3 and 7 contain amplified wild type gene (GTGT) products. Lane 2, 5 and 6 contain amplified wild type gene and pseudogene (ΔGT) products and lane 4, 8 and 9 contain amplified pseudogene products respectively. The result shows that the 2nd, 5th and 6th patients have both the wild type gene and pseudogene (GT/GTGT). The 3rd and 7th patients have the wild type gene (GTGT) and THE 4th, 8th and 9th patients have the pseudogene (ΔGT).
Genotypic Frequencies of wild type gene and pseudogene in Malaria Patients
It was found that in the Pakistani population, the frequency of the wild type gene in malarial patients (37.5%) was no higher than in healthy individuals (45%) with P = 0.30427. The combination of wild type gene and pseudogene(GTGT/ ΔGT) was equally prevalent in malarial patients (39.8%) as it was in healthy individuals (40%) with P = 0.99999,while the pseudogene (ΔGT) was also slightly different among healthy individuals (15%) as compared to malarial patients (22.7%) with P = 0.19263 which is shown in table 4. There was no significant association found because the P values was greater than 0.05.
Table 4: Show the association of NCF1 gene with malaria
| NCF1 gene | Control (n = 100) | Frequency (%) | Patients (n = 88) | Frequency (%) | P value |
| GTGT | 45 | 45 | 33 | 37.5 | 0.30427 |
| GT | 15 | 15 | 20 | 22.7 | 0.19263 |
| GTGT/GT | 40 | 40 | 35 | 39.8 | 0.99999 |
Discussion
In this study the association of a wild type gene and a pseudogene with malarial infection, which affects the hepatic, haematological and respiratory systems was investigated.
There was no association found between the wild type gene and pseudogene and the severity of malaria. The innate immune mechanisms that have been proposed to kill malaria parasites are those mediated by ROS and RNS, especially NO and ONOO-, both generated early during infection prior to the activation of adaptive immune mechanisms and later as components of the effector arm of the adaptive immune response11, 12. The parasite-killing role for these molecules has often been conflicting, especially when looking at in vitro and in vivo studies13. It was confirmed that neither RNS nor ROS are essential for the elimination of blood stage malaria parasites14. It was also shown in other studies that on occasion the generation of ROS via NADPH oxidase does kill blood stage malaria parasites, which is a controversial finding. A possible explanation of the discrepancies between Brad et al. and those of Sanni et al. is that these Plasmodium parasites differ in their susceptibility to the action of ROS, with P. yoelii and P chabaudi being more resistant than P. berghei. ROS might be incapable of killing blood stage malarial parasites for several reasons:
(i) The in vivo ability to kill malaria parasites may be masked by the antibody response of the infected host, and (ii) the killing mechanisms mediated by these molecules may function in a redundant fashion15. The present data does not confer the association of the wild type gene and pseudogene with the severity of malaria but there is a need for further study involving a larger population. However within a group of children with severe malaria during the acute disease, a weak association of the wild type gene /pseudogene (ΔGT/GTGT) ratio with ROS production in whole blood was found. It has been suggested that the wild type gene/ pseudogene (ΔGT/GTGT) ratio influences the expression levels of ROS16. However, no influence of the wild type gene /pseudogene ( ΔGT/GTGT) ratio on Plasmodium falciparum malarial infection was detected, although it was previously shown that ROS production plays a role in parasite clearance as well as in the pathology of the disease17, 9. As for parasitic diseases, in humans there has been only one study conducted so far which examined the putative genetic associations of the wild type gene and pseudogene (ΔGT/GTGT) ratio with malaria18.
|
Competing Interests None declared Author Details SHAKIRULLAH, M.Phil student of National University of Science and Technology (NUST) Center of Virology & Immunology (NCVI), Sector H-12 Islamabad MUHAMMAD ARSHAD, Associate professor in National University of Science and Technology SOHAIL AFZAL, M.Phil student of National University of Science and Technology NUST Center of Virology & Immunology (NCVI), Sector H-12 Islamabad. BAHADAR ZAIB, M.Phil in biotechnology SABA HAQ, NUST Center of Virology & Immunology (NCVI), Sector H-12 Islamabad. CORRESPONDENCE: Shakirullah, M.Phil student of National University of Science and Technology (NUST) Center of Virology & Immunology (NCVI), Sector H-12 Islamabad Email: shakir_pharmacist@yahoo.com |
References
- Volkman SK, Barry AE, Lyons EJ et al. Recent origin of Plasmodium falciparum from a single progenitor. Science 2001; 293:482–484.
- Singh B, Kim Sung L, Matusop A. A large focus of naturally acquired Plasmodium knowlesi infections in human beings. Lancet 200; 363 (9414): 1017–24.
- Fong YL, Cadigan FC, Coatney GR. A presumptive case of naturally occurring Plasmodium knowlesi malaria in man in Malaysia. Trans. R. Soc. Trop. Med. Hyg 1971; 65 (6): 839–40.
- Babior, B.M. NADPH oxidase: an update. Blood 1999; 93 (5): 1464–1476.
- Gorlach A, Lee P, Roesler J, et al. The p47-phox pseudogene carries the most common mutation causing p47-phox de?cient chronic granulomatous disease. J Clin Invest 1997; 100(8):1907–1918.
- Francke U, Hsieh C-L, Foellmer B, et al. Genes for two autosomal recessive forms of chronic granulomatous disease assigned to 1q25 (NCF2) and 7q11.23 (NCF1). Am J Hum Genet 1990; 47:483–492
- Chanock SJ, Roesler J, Zhan S, et al. Genomic structure of the human p47-phox (NCF1) gene. Blood Cells Mol Dis 2000; 26:37– 46.
- Greve B, Lehman LG, Lell B, et al. High oxygen radical production is associated with fast parasite clearance in children with Plasmodium falciparum malaria. J Infect Dis 1999; 179:1584-1586.
- Greve B, Kremsner PG, Lell B, et al. Malarial anaemia in African children associated with high oxygen radical production. Lancet 2000; 355(3):40-41.
- Uhlemann AC, Szlezak NA, Vonthein R, et al. DNA phasing by TA dinucleotide microsatellite length determines in vitro and in vivo expression of the gp91phox subunit of NADPH oxidase and mediates protection against severe malaria. J Infect Dis 2004;189 (12):2227-2234.
- Clark RA, Malech HL, Gallin JI, et al. Genetic variants of chronic granulomatous disease: prevalence of deficiencies of two cytosolic components of the NADPH-oxidase system. N Engl J Med 1989; 321;160:647-52.
- Taylor-Robinson A. W, Phillips R. S, Severn A, et al. The role of TH1 and TH2 cells in a rodent malaria infection. Science 1993; 260:1931–1934.
- Mohan, K., and. Stevenson M. M. Acquired immunity to asexual blood stages. In I. W. Sherman (ed.), Malaria: parasite biology, pathogenesis, and protection. American Society for Microbiology, Washington,D.C 1998; p. 467–493
- Brad M, Gillman, Batchelder J, et al. Suppression of Plasmodium chabaudi Parasitemia Is Independent of the Action of Reactive Oxygen Intermediates and/or Nitric Oxide 2004;72(11); p. 6359–6366
- Weidanz W. P, Kemp J. R, Batchelder J. M, et al. Plasticity of immune responses suppressing parasitemia during acute Plasmodium chabaudi malaria. J. Immunol 1999; 162:7383–7388.
- Heyworth PG, Cross AR, Curnutte JT. Chronic granulomatous disease. Curr Opin Immunol 2003; 15:578-584.
- Rosen GM, Pou S, Ramos CL, et al. Free radicals and phagocytic cells. FASEB J 1995; 9 (2):200-209.
- Bernhard G, Peter H, Reinhard V, et al. NCF1 gene and pseudogene pattern: association with parasitic infection and autoimmunity. Malaria Journal 2008; 251(7);1475-2875

The above article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.




