Aorto-enteric fistulas: a cause of gastrointestinal bleeding not to be missed
Louise MacDougall, John Painter, Terry Featherstone, Claus Overbeck, Shyju Paremal and Suvadip Chatterjee
Cite this article as: BJMP 2010;3(2):317
|
|
Abstract Aorto-enteric fistulas are a rare cause of gastrointestinal (GI) bleeding. The high mortality associated with this condition and relatively low incidence make this a diagnostic and management challenge. This case report describes a classic presentation of such a case along with a discussion on the diagnosis and treatment of this condition. We hope that this will be a clinical reminder to all physicians particularly those involved in managing GI hemorrhage in an acute medical take.
|
Clinical Presentation
A 87-year old man was referred to hospital with a five day history of lethargy and increased urinary frequency. He denied symptoms of gastrointestinal bleeding or abdominal pain. His past medical history included diabetes mellitus, chronic kidney disease, peripheral vascular disease and surgery for repair of ruptured aortic aneurysm 6 weeks ago. Systemic examination, including per rectal examination, was normal. Haemoglobin was 83g/L and C-reactive protein was 148 (Normal <5). Twelve hours after admission he developed pyrexia (37.8 degree) accompanied with tachycardia (103 beats per minute) and hypotension (BP 87/43). Soon afterwards, he had a small amount (<50 mls) of fresh haemetemesis. He also complained of lower back pain and clinical examination revealed tenderness in the left iliac fossa. He was cross-matched for blood and initiated on intra-venous fluids. As his Rockall score was six an urgent oesophago-gastro-duodenoscopy (OGD) was planned. Over the next few hours he complained of increasing central abdominal pain and had several episodes of melaena. In view of the history of recent aortic surgery and current GI bleed the possibility of aorto-enteric fistula (AEF) was considered. An urgent contrast CT scan of the abdomen (Figure 1) was therefore arranged prior to OGD.
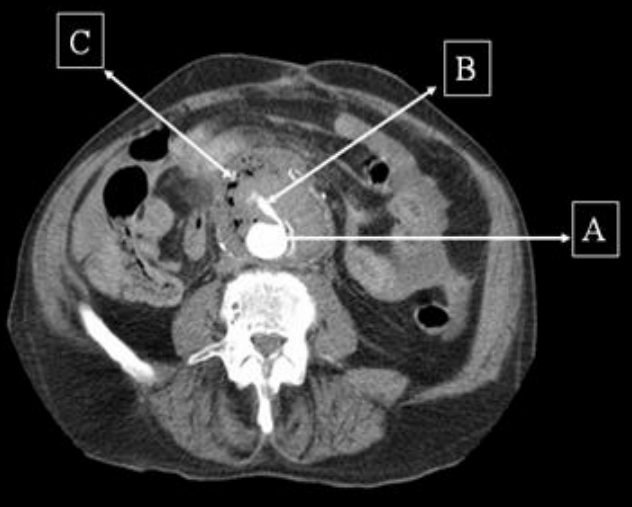
Figure 1: Contrast CT scan demonstrating the aorta (A) with extravasation of contrast (B) and a large collection (C) around it with trapped air suggestive of infection.
Contrast computed tomogram (CT) scan of the abdomen revealed an inflammatory soft tissue mass anterior to the infra-renal aortic graft with pockets of gas and leakage of contrast into it. These findings were suggestive of an AEF. The patient was informed of the diagnosis of AEF and the need for emergency surgical repair to which he consented. During the operation the vascular surgeons found that the duodenum was adherent to the aortic graft with evidence of fistulisation and infection, thus confirming the diagnosis. Although operative repair appeared to be successful, the patient continued to bleed on the table due to disseminated intravascular coagulation and died twenty fours after admission.
Discussion
AEF is defined as a communication between the aorta and the GI tract.1 The diagnosis of AEF should be considered in every patient with a GI bleed and a past history of aortic surgery.2 Our case patient had had emergency repair of a ruptured aortic aneurysm with a prosthetic graft 6 weeks prior to his current admission.
AEFs are a rare cause of gastro-intestinal (GI) hemorrhage. AEFs can be primary or secondary. Primary AEF (PAEF) is a communication between the native aorta and the GI tract.1 The incidence of PAEF ranges from 0.04 to 0.07%.3 PAEFs commonly arise from an abdominal aortic aneurysm of which 85% are atherosclerotic.1
Secondary AEFs (SAEF) are an uncommon complication of abdominal aortic reconstruction.4 The incidence of SAEF ranges from 0.6% - 4%.5 Generally two types of SAEFs have been described. Type 1, termed as true AEF develops between the proximal aortic suture and the bowel wall. These usually present with massive upper GI hemorrhage.4 Type 2, or the paraprosthetic–enteric fistula does not develop a communication between the bowel and the graft and accounts for 15% to 20% of SAEFs.4 In this type of fistula, bleeding occurs from the edges of the eroded bowel by mechanical pulsations of the aortic graft. Sepsis is more frequently associated with this type of AEF (75% of cases).4 The mean time interval between surgery and presentation with SAEF is about 32 months6 but the time interval can vary from 2 days to 23 years.7 AEFs can involve any segment of the GI tract but, 75% involve the third part of the duodenum and the affected part is generally proximal to the aortic graft.8
The pathogenesis of AEF is not fully understood but two theories exist. One theory suggests repeated mechanical trauma between the pulsating aorta and duodenum causes fistula formation and the other suggests low-grade infection as the primary event with abscess formation and subsequent erosion through the bowel wall.9 The latter theory is felt to be most likely. The majority of grafts show signs of infection at the time of bleeding and up to 85% of cases have blood cultures positive for enteric organisms.10
The main symptom of AEF is GI bleeding. Secondary AEFs have been traditionally said to present with a symptom triad (as in our patient) of abdominal pain, GI bleeding and sepsis; however, only 30% of patients present in this manner.11 Patients often have a “herald bleed” which is defined as a brisk bleed associated with hypotension and hematemesis that stops spontaneously followed by massive gastro-intestinal haemorrhage in 20% – 100% of patients.8 Sometimes the GI bleeding can be intermittent.
The commonest investigations for diagnosis of AEFs are OGD, conventional contrast CT scan and angiography.12 OGD is often the initial investigation, as in any upper GI bleed mainly because of lack of clinical suspicion of the diagnosis. The endoscopic findings vary from those of a graft protruding through the bowel wall to fresh bleeding in distal duodenum to that of an adherent clot or extrinsic compression by a pulsating mass with a suture line protruding into the duodenum.13 Less than 40% of patients have signs of active bleeding at OGD.8 Conventional CT with contrast is widely available and most commonly performed to diagnose AEFs. Perigraft extravasation of contrast is a pathognomic sign of AEF and this may be associated with signs of graft infection i.e perigraft fluid and soft tissue thickening along with gas.12 Multi-detector CT and MRI are more sensitive diagnostic imaging tools with MRI now being used mainly in patients with renal failure to avoid the use of contrast.12
PAEFs can be treated with endovascular stent placement in selected cases especially in those who cannot tolerate emergency surgery.12 The treatment of choice in SAEFs is graft resection and establishment of an extra-anastomotic circulation with repair of the duodenal wall although overall survival rates vary from 30% to 70%.13
Conclusion
SAEFs are a catastrophic complication of aortic surgery. AEFs are relatively rare and need a high index of suspicion in the appropriate clinical situation in order to diagnose this condition. Left untreated they are universally fatal. Surgical repair carries a very high mortality.
|
Competing Interests None declared Author Details Louise MacDougall, John Painter, Suvadip Chatterjee, Department of Gastroenterology, Sunderland Royal Hospital. United Kingdom. Terry Featherstone, Department of Radiology, Sunderland Royal Hospital. United Kingdom. Claus Overbeck, Department of Vascular Surgery, Sunderland Royal Hospital. United Kingdom. Shyju Paremal, Department of Medicine, Sunderland Royal Hospital. United Kingdom. CORRESPONDENCE: Dr Suvadip Chatterjee, MRCP(UK), MRCP(Ireland), MD, Specialist Registrar in Gastroenterology. Sunderland Royal Hospital. Kayll Road. Sunderland, SR4 7TP Email: suvadip_chatterjee@yahoo.com |
References
1.Ihama Y, Miyazaki T, Fuke C, Ihama Y, Matayoshi R, Kohatsu H, Kinjo F. An autopsy case of a primary aortoenteric fistula: a pitfall of the endoscopic diagnosis. World Journal of Gastroenterology 2008 August 7; 14(29):4701-4704.
2.Asfoor A M A, Nair G R. Secondary Aorto-duodenal fistulas. Bahrain Medical Bulletin Vol29, No 2,June 2007 : 1- 6.
3. Saers SJ, Scheltinga MR. Primary aortoenteric fistula.Br J Surg 2005;92:143 – 152.
4.Mohammadzade M A, Akbar H M. Secondary Aortoenteric fistula. Medscape General Medicine.2007; 9(3):25: 1-4.
5.Elliot JP, Smith RF, Sizlagyi DE. Aorto-enteric and paraprosthetic enteric fistula. Problems of diagnosis and management. Arch of Surg.1974;108:479.
6. Bastounis E, Papalambros E, MermingasV, Maltezos C, Diamantis T, Balsa P. Secondary aortoduodenal fistulae. J Cardiovasc Surg. 1997; 38: 457 – 464.
7.Shindo S, Tada Y, Sato O, et al. A case of aortoenteric fistula occurring 27 years after aorto-femoral bypass surgery, treated successfully by surgical management. Surg Today.1993;23: 993-997.
8.Busuttil SJ, Goldstone J. Diagnosis and management of aortoenteric fistulas. Semin Vasc Surg. 2001;14: 302 – 311.
9.Bussetil RW,Reese W,Baker JD,et al.Pathogenesis of aortoduodenal fistula, experimental and clinical correlates. Surgery. 1979;85:1-12.
10. Rosenthal D, Deterling Jr RA, O’Donnel Jr TF, et al. Positive blood culture as an aid in the diagnosis of secondary aortoenteric fistula. Arch Surg. 1979;114: 1041 -4.
11. Lau H, Chew DK, Gembarowicz RM, Makrauer FL, Conte M. Secondary aortoduodenal fistula. Surgery. 2001;130: 526-527.
12. Odemis B, Basar O, Ertugul I, Ibis M, Yuksel I, Ulcar E ,Arda K. Detection of an aorto-enteric fistula in a patient with intermittent bleeding. Nature Clinical Practice Gastroenterology and Hepatology.2008 (5):226 – 230.
13. Champion MC, Sullivan SN, Coles JC, Goldbach M, Watson WC. Aortoenteric fistula. Incidence, presentation, recognition and management. Ann Surg 1982(3): Vol 195; 314-317.

The above article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.




