Acanthosis Nigricans in Pre-diabetic states
James Paul Pandarakalam
Cite this article as: BJMP 2018;11(1):a1105
|
|
Abstract Abstract: The high incidence of type 2 diabetes mellitus has become for many a heavy penance for enjoying the luxuries of modern living. Today’s western life style is characterised by sedentary habits and high-calorie food intake, which are contributory factors for this condition. If the prediabetic stage if identified early, it may be prevented from progressing into full diabetes. A significant percentage of occurrences of type 2 diabetes may be reversed if loss of weight and maintenance of a healthy body mass index (BMI) is achieved. At the same time as the life-style changes, the use of atypical antipsychotic medication is resulting in an increase in a specific metabolic syndrome among the psychiatric population. Along with other symptoms that herald this disease, darkened patches on the skin may be a warning signal to alert potential sufferers from diabetes to take precautionary measures. If acanthosis nigricans is proven to have autoimmune components, the same could be true of Diabetes Mellitus Type-ii. Keywords: Key words: overweight, Acanthosis nigricans, insulin resistance, autoimmunity, risk factors. |
The identification of dark patches on the skin may be the first indication of type 2 diabetes mellitus (DM type 2). DM type 2 is a complex heterogeneous group of metabolic conditions characterised by elevated levels of serum glucose. Causative factors are impairment in both insulin action and insulin secretion. The darkening of the skin is usually evident on the hands and feet, in folds of skin, along the neck, and in the patient’s groin and armpits.1 The affected skin differs from that which surrounds it, and it feels velvety and also thicker. That skin may have hanging from it the small, soft, skin-coloured growths known as tags, and the area affected may be pruritic. This condition is a nonspecific dermatological disorder termed acanthosis nigricans (AN), which often occurs in patients with high insulin levels. Hud et al. (1992) found that 74% of the obese population exhibits AN.2The association of AN, skin tags, and diabetes mellitus due to insulin resistance – along with obesity in adolescents and young adults – is a well-defined syndrome.3,4,5
High-insulin levels in the blood may increase the body’s production of skin cells, many of which have increased pigmentation that gives the skin a darkened appearance – dark patches appear on the skin. These are often the outcome of insulin receptors in the skin being triggered, causing mutations of normal tissue that are dark in colour and/or irregular in shape. The condition may be an indication that the blood sugar is persistently high. The term ‘acanthosis nigricans’ was originally proposed by Unna et al. in 1891, but the first descriptions of it were made a year earlier by two researchers working independently of each other: Pollitzer and Janovsk.6 Kahn and colleagues tried to clarify the link between AN and insulin resistance in 1976.7 Eventually, its presence became established as an indicator of insulin resistance or diabetes mellitus in obese patients.8 In 2000, the American Diabetes Association formally accepted AN as such.9 It should be borne in mind that AN must not be considered a characteristic feature of DM type 2; it is not a condition that is developed by all those who suffer from the disease.
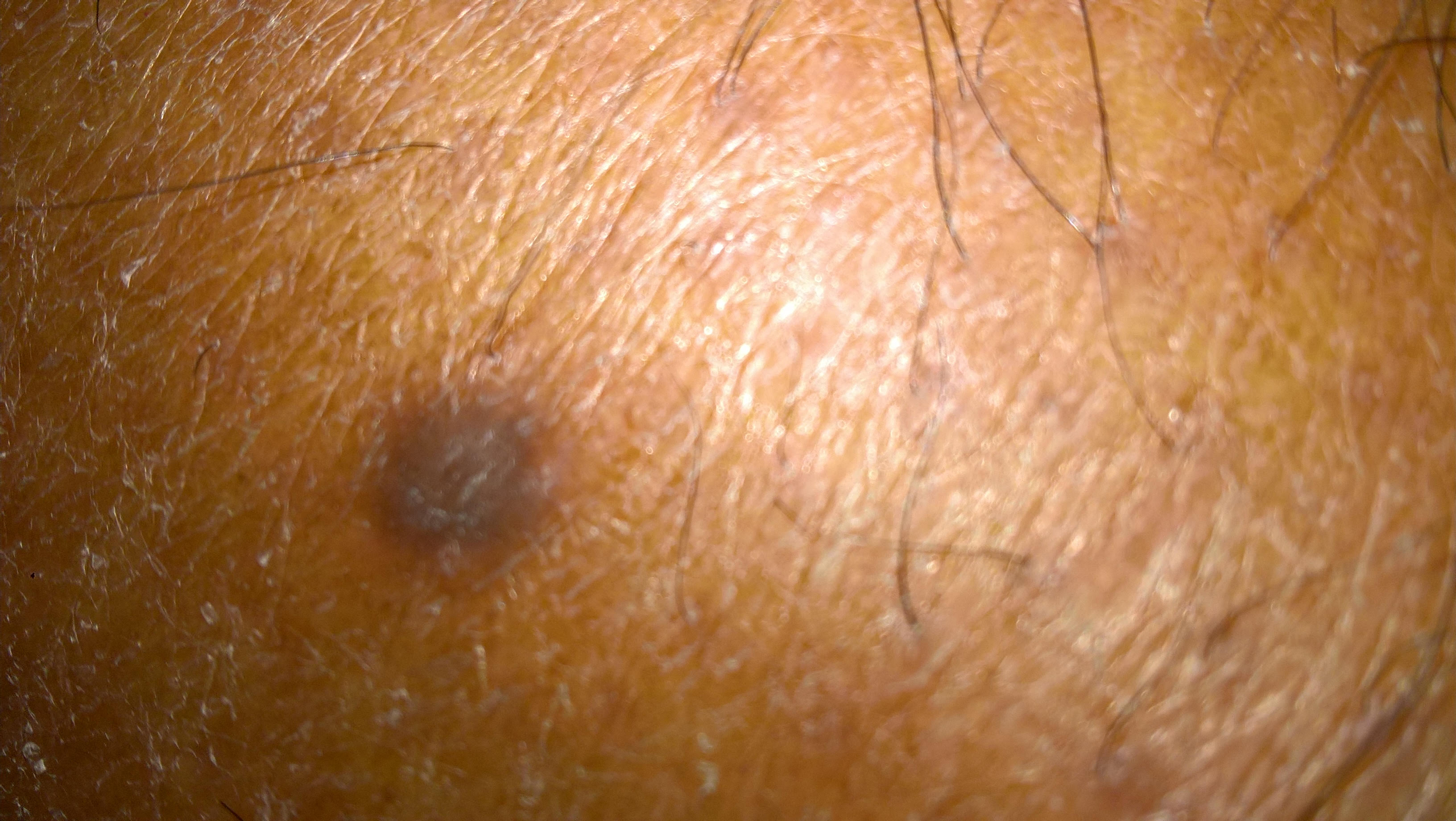
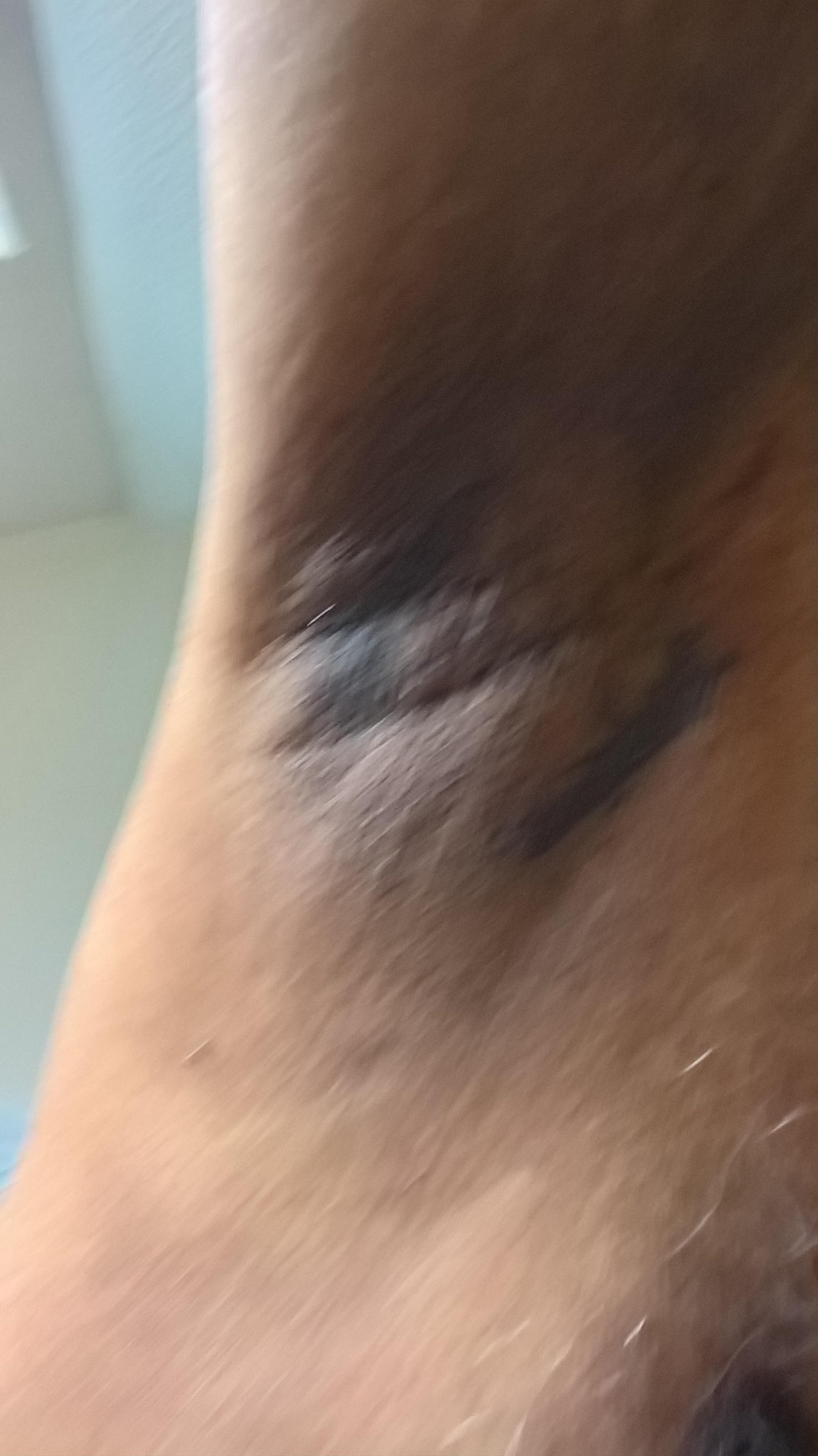
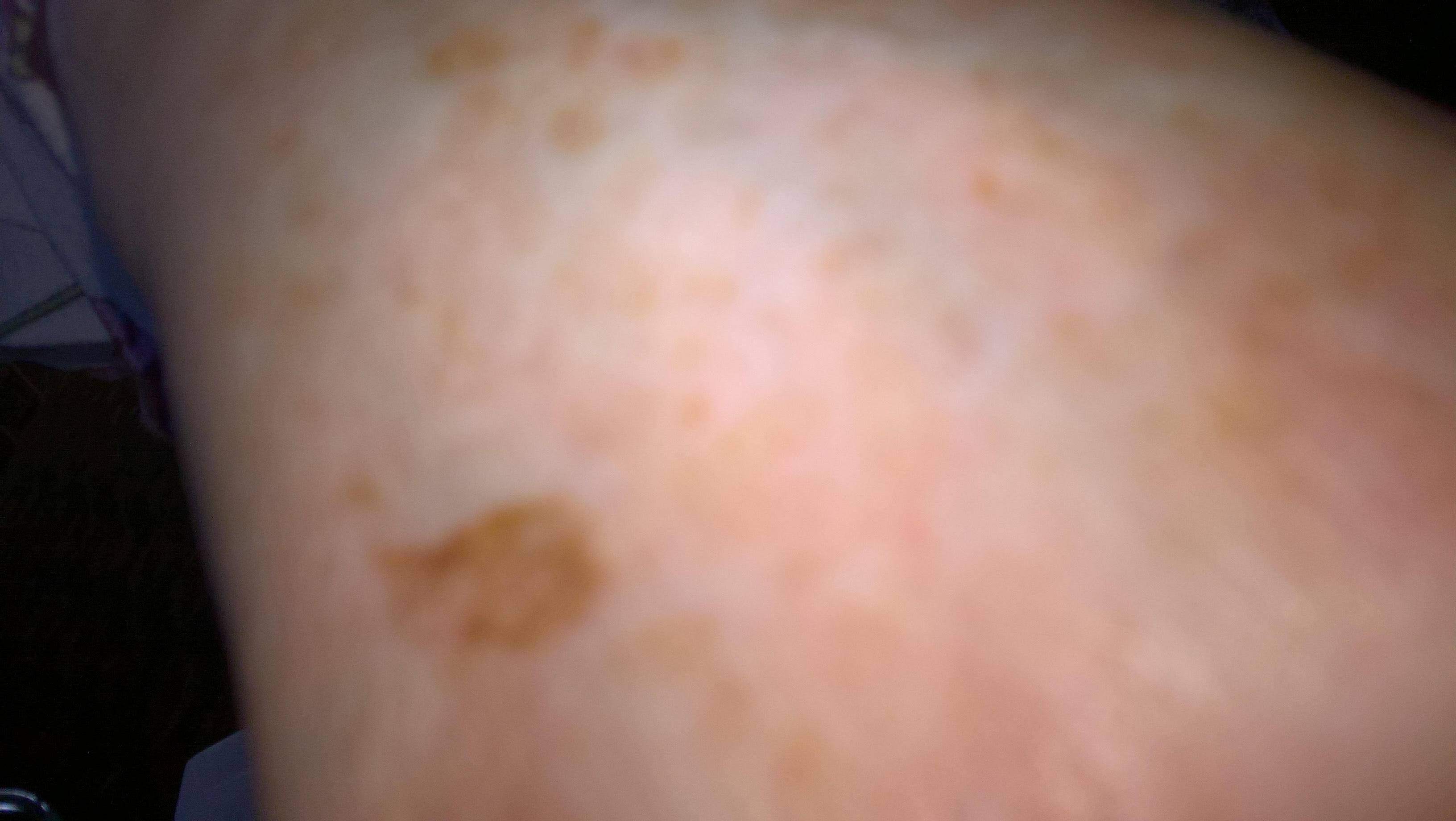
Figures 1,2,3 - Acanthosis Nigrans
Pathogenesis
Although the pancreas produces insulin in DM type 2, the body cannot make use of it efficiently. The outcome is a build-up of glucose in the bloodstream, which may lead to high levels of blood glucose and insulin. At low concentrations, insulin regulates the metabolism of carbohydrates, lipids, and protein and may promote growth by binding to ‘classic’ insulin receptors. High concentrations of insulin may stimulate keratinocyte and fibroblast proliferation through high-affinity binding to the insulin-like growth factor 1 (IGF-1) receptors.10 In obese patients elevated IGF-1 levels may contribute to keratinocyte and fibroblast proliferation;11 the binding stimulates the proliferation of keratinocytes and fibroblasts, which leads to AN.
To put this simply, AN is the outcome of a toxic effect of hyperinsulinemia. Excess insulin causes the normal skin cells to reproduce rapidly rate, and it has been demonstrated to cross the dermo-epidermal junction and reach keratinocytes. In those who have dark skin, these new cells have increased melanin. The higher level of melanin results in the creation of a patch of skin that is noticeably darker than the skin surrounding it. The presence of AN is therefore a strong indicator of increased insulin production and, therefore, it is also a predictor for future DM type 2.
When the occurrence of AN is recognised, a prediabetic person has the opportunity to become more alert to their symptoms and to take precautions in the form of diet restrictions and weight loss. This is because overweight people tend to develop resistance to insulin over time. If too much insulin is the cause of AN, it is relatively easy for the patient to counter it by changing to a healthier diet, taking exercise, and controlling their blood sugar. Obesity-associated AN may be a marker for higher insulin needs in obese women with gestational diabetes,12andAN has been shown to be a dependable early indicator for metabolic syndrome in paediatric patients.13
Autoimmunity?
Unknown autoantibodies other than insulin receptors have been implicated in AN, which may explain the effectiveness of cyclosporine treatment. Kondo and colleagues identified a very rare occurrence – without type B insulin resistance – of generalised AN with Sjögren's syndrome and systemic lupus erythematosus-like features.14Theirs was the first report of generalised AN involving an area from the mucosa of the larynx to the esophagogastric junction, accompanied by autoimmune disorder (AD) responding to systemic immunosuppressive therapy. AN skin lesions and mucosal papillomatosis were medicated with oral cyclosporine A and were accompanied by lower autoantibody titres. That was an outcome of the development of antibodies to insulin receptors in AD such as systemic lupus erythematosus.15
Raymond et al. have reported on the association of AN with disordered immunoreactivity.16 The onset of AN may precede a variety of classic ADs, and different categories of ADs may be present at the same time. If AN is an AD, DM type 2 may also represent a slow and subtle autoimmune process. AN and DM type 2 then become two different expressions of the same disease process, but the former is apparently benign and the latter is ultimately potentially fatal. Autoimmunity is a well-known pathogenic component in DM type 2. The assumption that its pathogenesis encompasses autoimmune aspects is increasingly recognised. That is based on the presence of circulating autoantibodies against β cells, self-reactive T cells, and also on the glucose-lowering efficacy in DM type 2of some immunomodulatory therapies.17 The autoimmune hypothesis of AN has the potential to modify the direction of DM type 2 research.
The symptoms of ADs are inconstant and this is in divergence to the mechanisms of antigen recognition and effector function that are alike in the response to pathogens. 18 The symptoms basically depend on the triggering autoantigen and the target tissue. In certain conditions, autoantibodies function as receptor antagonists and in other situations, they function as receptor agonist. Autoantibodies of both types can be made against insulin receptor. When they serve as antagonists as in DM Type 2, the cells of patients are unable to take up glucose, the consequence is hyperglycaemia whereas in patients with agonistic antibodies, cells deplete blood glucose resulting in hypoglycaemia.18 One wonders whether AN may be an early by-product of such an autoimmune process.
Vitiligo which is the result of depigmentation of the skin is in fact an opposite disorder to AN. Vitiligo is recognised as an AD.19Thyroid disorders, particularly Hashimoto thyroiditis and Graves’ disease, other endocrinopathies, such as Addison disease, diabetes mellitus, alopecia areata, pernicious anaemia, inflammatory bowel disease, psoriasis, and autoimmune poly-glandular syndrome are all associated with vitiligo. 20 Kakourou et al identify that Hashimoto's thyroiditis is 2.5 times more frequent among children and adolescents with vitiligo than in a healthy age- and sex-matched population and it usually follows the onset of vitiligo. 21
As in the case of other ADs, vitiligo susceptibility may involve both target organ-specific genes and immune response genes. 22 The autoimmune theory suggests alteration in humoral and cellular immunity in the destruction of melanocytes of vitiligo. 23. Vitiligo lesions have an infiltrate of inflammatory cells, particularly cytotoxic and helper T-cells and macrophages;histological evidences further back up an autoimmune aetiology. 24 Like AN, Vitiligo is thus gene linked; immunity derangements may be providing the matrix and genes are the craftsmen in both conditions.
Vitiligo occurs more commonly in DM Type 1. A few recent studies have revealed its increased incidence in DM Type 2. 25 These may be isolated case studies, but offer new insight into the pathogenesis of DM Type 2. There is a logical thread running between the autoimmune assertion of AN and its depigmentation counterpart (vitiligo) which is recognised as an AD. If AN is proven as an AD, the AD hypothesis of DM Type 2 becomes more binding. The autoimmune process of AN warrants further consideration and further study is needed to confirm or falsify the hypothesis of an autoimmune spectrum disorder between AN, vitiligo and DM Type 2.
Even though the common assumption that bacteria flora occupying human body outnumber the body cells has been proven wrong, their revised ratio of 1:1 is still astounding.26 The exact role of the resident microbial colony in human body is unclear. There are less ADs observed among the hunters’ tribal population of Tanzania whose faecal matter contain more varieties of microbes than people in developed countries. 27,28 This is an observation that need further verification. It is possible that the occupied microbial army may be maintaining the harmony among the human body cells from attacking each other and serving as moderators. Now that anti-autoimmune activity in molecules produced by parasites have been confirmed in haematology lab, these findings may have clinical significance. The aetiology of ADs is multifactorial. Genetic, environmental, hormonal, psychological stress and immunological factors are all considered important in their development. I content that the clue to the mechanism of development of certain ADs and ways of counteracting them may be embedded in the bacterial colony and their interaction with human cells.
International studies
A pilot study by Bhagyanathan and colleagues demonstrated that children with AN have a high incidence of insulin resistance.29 They posit that the detection of insulin resistance in children may present an opportunity to prevent the onset of microvascular changes before the development of DM type 2. Once DM type 2 by hyperglycemia is diagnosed it may be too late for that. Insulin resistance is one of the mechanisms involved in the pathogenesis of DM type 2; therefore, early recognition of insulin resistance is paramount in the prevention or delay of the onset of diabetes. Their study was of 62% of children who had AN alongside high insulin resistance. In children with AN and a high BMI, the incidence of insulin resistance was about 80%. This is evidence that the easily detectable symptoms are of value in the screening of children who are at high risk of developing DM type 2. Bhagyanathan et al. conclude that AN has potential as a screening method because those who have high insulin resistance as well as AN are at high of future DM type 2.
An earlier US study, by Brickman and colleagues, had yielded somewhat similar results.30 This involved 618 youths from different ethnic groups at nine paediatric practices. They were aged from 7 to 17 years. A survey was made of their demographics and their family history with regard to DM type 2, and their weight and height were also measured. AN was scored and digital photographs of their necks were taken. AN was identified in 19%, 23%, and 4% of the African American, Hispanic, and Caucasian youth respectively. It was also found in 62% of those studied who had a BMI greater than the 98th percentile. Using multiple logistic regression, the researchers found that the level of BMI, the presence of maternal gestational diabetes, female gender, and not being Caucasian, were all independently associated with AN. AN was common among the overweight young people and was associated with risk factors for glucose homeostasis abnormality. Brickman et al. concluded that identification of AN offers an opportunity to advise families about the causes and consequences of the condition. 30 That has the potential to motivate those with responsibility for the young people to encourage and effect healthy lifestyle changes that decrease the risk of the development of DM type 2 and cardiovascular disease.
In their research in India, Vijayan et al. determined that BMI, waist circumference and AN are three physical markers for the recognition of insulin resistance in children.31 They conducted a cross-sectional school-based study in a semi-rural environment in the state of Kerala, which has become known as the diabetic capital of the country. Their study encompassed 283 children between the ages of 10 to 17. The prevalence of insulin resistance was 35%; this estimate was arrived at by using a homeostasis model assessment of insulin resistance (HOMA-IR). Of the children studied, 30% had a waist circumference above the 75th percentile and 18.7% had a BMI above 85th percentile. AN was diagnosed in 39.6% of the population studied. A significantly high prevalence of insulin resistance was observed among the children with a waist circumference exceeding the 75th percentile, a BMI above the 85th percentile, or a diagnosis of AN. The most sensitive physical marker of insulin resistance was AN (90%) and the most specific was BMI (91%). By combining these parameters their sensitivity may be increased to 94% and their negative predictive value to 96%. Vijayan et al. conclude that these easily recognisable physical markers are an efficient warning of insulin resistance among children.
Acanthosis nigricans in different conditions
AN is not a disease, but a symptom of disease. A high prevalence has been observed recently, and there are a number of varieties. These include benign, obesity associated, syndromic, malignant, acral, unilateral, medication-induced, and mixed AN.32,33 It has been established that AN may occur in a number of conditions, and a brief discussion of these is appropriate for this paper. Different types of AN are listed in Table 1. It often appears gradually in the prediabetic state, but abruptly in malignancy.
AN may be triggered by a plethora of medications, such as birth control pills, human growth hormones, thyroid medications, and even some bodybuilding supplements. All these medications may cause changes in insulin levels. Medications used to ease the side effects of chemotherapy have also been linked to AN. In most cases, the condition clears up when the medications are discontinued. In rare cases, AN may be caused by gastric cancer (especially gastric adenocarcinoma) or an adrenal gland disorder such as Addison’s disease. Hypothyroidism, Cushing’s disease, and polycystic ovarian disease are also common causes of AN. 34,35
When AN is present without any identifiable cause in middle-aged and older patients with extensive skin findings, internal malignancy needs to be ruled out. AN has been reported in association with many kinds of cancer, by far the most common being an adenocarcinoma of gastrointestinal origin. In these patients it is a rapid-growing dermatological pigmentation disorder. The skin changes are typically more extensive and severe than those seen in benign AN. Findings may include thickening, unusual roughness and dryness, and/or potentially severe itching (pruritus) and irritation of the skin regions affected. Pigmentary changes may be more pronounced than those observed in benign AN and they are not restricted to areas of hyperkeratosis. Malignant AN is frequently associated with the mucous membranes and with distinctive abnormalities of the oral (mouth) region. For example, reports indicate that the lips and the back and sides of the tongue may have an unusually ‘shaggy’ appearance, sometimes with elevated, wart-like, non-pigmented tissue growths (papillomatous elevations). Malignant AN is also commonly characterised by wart-like thickening around the eyes, unusual ridging or brittleness of the nails, thickening of the skin on the palms of the hands, hair loss, and sometimes other symptoms. Investigators have reported that the development of malignant AN may occur as much as five years before the onset of other symptoms, although the time span before malignancy is typically of shorter duration.
Table 1. Different types of acanthosis nigricans
| 1. Obesity-associated acanthosis nigricans. Obesity-associated acanthosis nigricans, once labelled pseudo-acanthosis nigricans, is the most common type. Lesions may appear at any age but are most common in adulthood. The dermatosis is weight dependent, and lesions may completely regress with weight reduction. Insulin resistance is often present in these patients. It is slow growing. 2. Acral acanthosis nigricans. Acralacanthosis nigricans (acral acanthotic anomaly) occurs in patients who are otherwise in good health. Acral acanthosis nigricans is most common in dark-skinned individuals, especially those of African-American or sub-Saharan-African descent. The hyperkeratotic velvety lesions are most prominent over the dorsal aspects of the hands and feet, with knuckle hyperpigmentation often most prominent. 3. Unilateral acanthosis nigricans. Unilateral acanthosis nigricans, sometimes referred to as nevoid acanthosis nigricans, is believed to be inherited as an autosomal dominant trait. Lesions are unilateral in distribution and may become evident during infancy, childhood, or adulthood. Lesions tend to enlarge gradually before stabilising or regressing. 4. Generalised acanthosis nigricans. Generalised acanthosis nigricans is rare and has been reported in paediatric patients without underlying systemic disease or malignancy. 5. Syndromic acanthosis nigricans. Syndromic acanthosis nigricans is the name given to acanthosis nigricans that is associated with a syndrome. The type A syndrome and type B syndrome are special examples. 6. Hereditary acanthosis nigricans. Familial acanthosis nigricans is a rare genodermatosis that seems to be transmitted in an autosomal dominant fashion with variable phenotypic penetrance. The lesions typically begin during early childhood but may manifest at any age. 7. Drug-induced acanthosis nigricans. Drug-induced acanthosis nigricans, although uncommon, may be induced by several medications, including nicotinic acid, insulin, pituitary extract, systemic corticosteroids, and diethylstilbestrol. Rarely, triazinate, oral contraceptives, fusidic acid, and methyltestosterone have also been associated with it. 8. Malignant acanthosis nigricans. Malignant acanthosis nigricans, which is associated with internal malignancy, is the most concerning variant of acanthosis nigricans because the underlying neoplasm is often an aggressive cancer. 9. Mixed-type acanthosis nigricans. Mixed-type acanthosis nigricans refers to those situations in which a patient with one of the above types develops new lesions of a different ethology. |
Genetic links
It is worth noting that certain types of AN may be genetically linked.36 The interaction of genes and the environment is not clearly understood and the different variables of DM type 2 are not established. It is a heterogenous disorder and there is a general consensus that diabetic comorbidities may be the outcome of genetic and environmental susceptibilities.37,38,39,40,41 Such factors may have an influence independently or in combination with one another that brings about hyperglycaemic conditions.It would be interesting to explore the possibility of a link between the diabetic genes and the AN gene. DM type 2 may be potentiated by poor quality of insulin or decreased production of insulin, and the distinction between those manifestations is not well recognised. The controversy concerning the relative roles of insulin deficiency and insulin resistance in DM type 2 continues to be unresolved.42 Despite the early demonstration that obese people have elevated plasma insulin concentrations, many studies over the years have failed to control satisfactorily for the influence of obesity.43 Another difficulty with the interpretation of plasma insulin concentrations is that sustained hyperglycaemia may have detrimental effects on insulin secretion.44 Diabetes mellitus affects every cell of the body, and therefore it affects the beta cells of the pancreas in turn. The spiralling effect of hyperglycaemia adds to the malfunctioning of beta cells, and that results in impaired quantity and quality of insulin. Only a subset of diabetic patients shows AN, and other groups of obese diabetic patients do not develop AN. AN is linked with higher insulin production and obesity, whereas AN may not be present in diabetes with a reduced quantity of insulin. The presence of AN may serve as one of the biological markers to determine subtypes of DM type 2.
The incidence of AN varies in different races, which is evidence that AN may have a genetic contribution – indeed, it has been regarded by some as being strongly influenced by genetic factors. It is thought to be autosomal in nature. AN is common among African-Americans, Hispanics, and American Indians, but it is rare among white people.45,46 A study from the USA reports the prevalence of AN as 3% among Caucasians, 19% in Hispanics, and 28% in American Indians. 47 More recently, studies from Sri Lanka and south India show the prevalence of AN as high as 17.4% and 16.1% respectively in the adult population in general.48,49
Type 2 diabetes mellitus and schizophrenia
DM type 2 is relatively common among people who have mental health issues. Increased risk for cardiovascular disease and other serious illnesses related to insulin resistance – for example, certain epithelial cell carcinomas, AN, and polycystic ovary syndrome – are long-term concerns associated with the cluster of metabolic abnormalities stemming from insulin resistance. These are often referred to as the metabolic syndrome.50 Impaired action of insulin in patients with schizophrenia was reported over fifty-five years ago and later confirmed in Australia.51 The prevalence of DM type 2 in patients with schizophrenia was found to be higher than it was in the general population, even before antipsychotic medication was in widespread use.
The mechanisms underlying the relationship between schizophrenia and diabetes remain unexplained. The present author has argued in favour of the autoimmune hypothesis of a subset of schizophrenia.52,53 The proposal is that if AN is an AD, it may be co-existing with DM type 2, or the DM type 2 itself may even be an extension of the same autoimmune process. In other words, there may be a continuum of pathological process between AN and DM type 2. It follows that schizophrenia sufferers may have a predisposition to develop DM type 2; schizophrenia may even be considered as a clinical surrogate of DM type 2.
When AN occurs in a schizophrenic patient, they sometimes develop a delusional misinterpretation of the condition, such as that it is a result of skin cancer or even a manifestation of an external agency. Such situations may result in severe anxiety. Schizophrenia is frequently associated with poor lifestyle choices on the part of the patient, such as a diet high in fat, reduced levels of physical activity, and high rates of smoking-all of these may contribute to the development of a metabolic syndrome and insulin resistance.54,55 It is worth considering investigation into the early warning signs for DM type 2 – including the AN – before commencing a patient on antipsychotic drugs that lead to a metabolic syndrome.
It is now well recognised that patients treated with clozapine or olanzapine are more often classified as having DM type 2 or impaired glucose tolerance in comparison with patients treated with other second-generation antipsychotics. Clozapine increases the risk of diabetes if there is a history of pre-existing diabetes or a family history of diabetes. According to a US study, the risk is higher if the patient is African-American or of Hispanic origin. Such patients may need close blood sugar monitoring during the initiation of clozapine treatment. I contend that if a patient already has AN, weight-increasing antipsychotics should be avoided. Even though aripiprazole is the most metabolic-sparing agent among the second-generation antipsychotics, Manu et al. report a case of AN in a patient treated with it. That patient did have a family history of DM type 2, which adds to the interest of the case.56
Diagnosis and treatment
There is no specific treatment for AN. Treatment is directed towards the specific symptoms that are apparent in each individual. It should be borne in mind that such treatment may require the coordinated efforts of a team of medical professionals. Correcting the underlying disease improves the skin symptoms. Steps that may be taken, depending on what the disease is, include correcting hyperinsulinemia through diet and medication, encouraging the loss of weight in those with obesity-associated AN, removing or treating a tumour, and discontinuing a medication that causes AN. The control of obesity contributes significantly to reversing the whole process, essentially by reducing both insulin resistance and compensatory hyperinsulinemia. However, the pigmentary changes may persist. In drug-induced AN, offending medicines should be stopped. In hereditary AN, lesions tend to enlarge gradually before stabilising and/or regressing on their own.
For those with AN, the recommended treatment may include the use of certain synthetic, vitamin A-like compounds (retinoids). For individuals with malignant AN, disease management requires treatment by oncologists. Reports indicate that AN has improved with therapy used to treat underlying malignancies and has reappeared with tumour recurrences. Other treatment for this disorder is symptomatic and supportive. The treatments considered are used primarily to improve appearance, and include topical retinoids, dermabrasion, and laser therapy. The final outcome of AN varies depending on the cause of the condition. Benign conditions, either on their own or through lifestyle changes and/or treatment, have good outcomes. The prognosis for patients with malignant AN is often poor as the associated cancer is often advanced.
AN may be diagnosed on the basis of thorough clinical evaluation, identification of characteristic physical findings, a complete patient history including medication history, a thorough family history, and various specialised tests.57 The age at detection will vary, depending upon the form of AN present and on other factors. For example, benign forms of AN often become evident during childhood or puberty. It is less common for benign AN to be apparent at birth or to develop in adulthood. The latter cases most typically involve AN in association with obesity.
In individuals with skin changes that suggest AN, diagnostic assessment may include various laboratory tests. Examples are the glucose tolerance test and the glycated haemoglobin (HbA1c) test. Additional laboratory studies or other specialised tests may also be utilised in diagnosis in order to help detect or rule out certain other underlying disorders – including a number of endocrine and autoimmune conditions – that may be associated with AN. In addition, in some instances, particularly where the patient presents with signs suggestive of malignant AN, testing may include biopsy and microscopic evaluation of small samples of skin tissue affected.
The onset of malignant AN usually occurs after the patient reaches 40 years of age. Various factors may be indicative of malignant AN in association with an underlying cancer. These include symptom onset in adulthood that is not associated with the use of particular medications, obesity, a positive family history, and certain underlying disorders known to be associated with AN. It is rare for malignant AN to develop during childhood. In such instances, warning signs may include skin changes that progress rapidly and also involvement of the mucous membranes.58
AN may be metaphorically linked to the dark pigmentation that appears on the skin of the ripe Sharon fruit. Sharon fruit is the trade name for a variety of persimmon that is grown in Israel. In the fruit the dark patches on the ripe and sugary fruits are the result of condensed tannins. Insulin-resistant AN may be referred to as the Sharon fruit sign in order to emphasise the diagnostic value of the condition. It has been suggested that the official terminology for AN is inappropriate for a significant warning of an increasingly common disease for which early diagnosis is imperative. Because the complex name may have a negative impact on its identification by both clinicians and patients, a less formal term is in use among some of those who are concerned with patient care. It must be borne in mind that AN, otherwise Sharon fruit sign, manifests only in those with the insulin-resistant condition and should not be considered a characteristic feature of DM Type 2. Identification of the Sharon fruit sign may be helpful in the early diagnosis of DM type 2.
Discussion
Diabetes puts an enormous burden on patients, their families, and the health-care system. Detection of the disease at an early state using physical markers and instituting preventive measures will reduce the economic strain on society to a great extent. According to the latest global data from the World Health Organization (2016), an estimated 422 million adults are living with DM type 2 and diabetes prevalence is increasing rapidly.59 In 2013 the International Diabetes Federation estimated that 381 million people were living with diabetes.60 That number is anticipated to almost double by 2030.61 About 3.8 million people in the UK have DM type 2, and the charity Diabetes UK has made predictions that it may become as high as 6.2 million by 2035/36.
Most often a diagnosis of DM type 2 is made only when such symptoms as loss of weight, polydipsia, and polyurea have become manifest. By that time the damage to the body may have already come about. Complications arising from diabetes cover the entire area of medical science, so early detection is crucial. Intervention at the prediabetic stage helps to arrest the progress of this condition. AN may herald DM type 2, endocrinopathies, and malignancies. This cutaneous disorder is easily detectable and highly useful in the early detection of the disorders associated with it. Early screening for AN in preadolescent and adolescent people would provide a relatively simple, inexpensive, and non-invasive tool for identifying those young people who have hyperinsulinemia and could benefit from early intervention. That would prevent the development of DM type 2. Young people tend to be reluctant to undergo traditional screening measures and definitive diagnostic tests as they find them invasive and unpleasant.
A sedentary life style and unhealthy dietary habits – as well as the side effects of antipsychotics – make chronically ill psychotic patients more vulnerable to DM type 2 than the general population. Long-standing detained patients in particular are restricted in their mobility and may become more prone to obesity and insulin resistance. It is not clear whether the pathogenesis of psychosis itself has a diabetogenic effect. It is evident that because of the high incidence of DM type 2 among mental health service users, psychiatrists need to become more alert in the diagnosis, management, and prevention of the complications of DM type 2.
|
Competing Interests None declared Author Details Dr James Paul Pandarakalam, Consultant Psychiatrist, Northwest Boroughs Health Care NHS Foundation Trust, Warrington WA2 8WN, UK CORRESPONDENCE: Dr James Paul Pandarakalam, Consultant Psychiatrist, Northwest Boroughs Health Care NHS Foundation Trust, Hollins Park Hospital & AFG Rehab Hospitals, Winnick Lane, Warrington WA2 8WN, UK Email: jpandarak@hotmail.co.uk |
References
- James WD, Berger TG, Elston DM. Andrew's Diseases of the Skin: Clinical Dermatology, 11th Edition, Saunders Elsevier Publications, Canada, 2011; pp. 494–5.
- Hud JA, Jr, Cohen JB, Wagner JM, Cruz PD., Jr Prevalence and significance of acanthosis nigricans in an adult obese population. Arch Dermatol. 1992; 128:941–4
- Yosipovitch G, DeVore A, Dawn A. “Obesity and the skin: Skin physiology and skin manifestations of obesity.” J Am Acad Dermatol 2007; 56:901-16
- Stoddart, M. L., Blevins, K. S., Lee, E. T., & Blackett, P. R. Association of acanthosis nigricans with hyperinsulinemia compared with other selected risk factors for type 2 diabetes in Cherokee Indians: the Cherokee Diabetes Study [Abstract]. Diabetes Care 2002; 25(6), 1009-1014.
- Hermanns-Lê T, Scheen A, Piérard GE.Am J Clin Dermatol. Acanthosis nigricans associated with insulin resistance: pathophysiology and management. 2004;5(3):199-203.
- rke JP, Hale DE, Hazuda HP, Stern MP. A quantitative scale of acanthosis nigricans. Diabetes Care. 1999; 22:1655–9.
- hn CR, Flier JS, Bar RS, Archer JA, Gorden P, Martin MM, Roth J. The syndromes of insulin resistance and acanthosis nigricans. Insulin-receptor disorders in man. N Engl J Med. 1976; 205(14):739-745.
- Schilling WHK, Crook MA. Cutaneous stigmata associated with insulin resistance and increased cardiovascular risk. Int J Dermatol. 2014; 53:1062-9.
- Sinha S, Schwartz RA. Juvenile acanthosis nigricans. J Am Acad Dermatol. 2007;57(3):502-8.
- Wertheimer panel,EfratMeiravTrebicz,ToraEldar,MarinaGartsbeinmSharonNofeh-Moses,‡ TamarTennenbaum. Differential Roles of Insulin Receptor and Insulin-Like Growth Factor-1 Receptor in Differentiation of Murine Skin Keratinocytes. Journal of Investigative Dermatology 2000; 115 (1): 24-29
- Higgins SP, Freemark M, Prose NS. Acanthosis nigricans: a practical approach to evaluation and management. Dermatol Online J. 2008 Sep; 15. 14(9).
- Mlinar B, Marc J, Janez A, Pfeifer M. Molecular mechanisms of insulin resistance and associated diseases. Clin Chim Acta. 2007; 375:20–35.
- Grundy SM. Hypertriglyceridemia, insulin resistance, and the metabolic syndrome. Am J Cardiol. 1999; 83:25F–9.
- Y. Kondo, N. Umegaki, M. Terao, H. Murota, T. Kimura, and I. Katayama. A Case of Generalized Acanthosis Nigricans with Positive Lupus Erythematosus-Related Autoantibodies and Antimicrosomal Antibody: Autoimmune Acanthosis Nigricans? Case Rep Dermatol. 2012; 4(1): 85–91. Published online 2012 Mar 30. doi: 10.1159/000337751
- Pavithran K, Karunakaran M, Palit A. Disorders of keratinization. In: Valia RG, Ameet RV, editors. IADVL Textbook of Dermatology. 3rd ed. Mumbai: Bhalani Publishing House; 2008; pp. 1009–11.
- Raymond A. Sturner, Stephen Denning, Patricia Marchase. Acanthosis Nigricans and Autoimmune Reactivity. JAMA. 1981;246(7):763-765. doi:10.1001/jama.1981.03320070047024
- Itariu B.K. Stulnig T.M. Autoimmune Aspects of Type 2 Diabetes Mellitus - A Mini-Review. Gerontology 2014; 60:189-196.
- Parham Peter.The Immune System. London: Garland science, 2009.
- Saleem K, Azim W. Association of Vitiligo with Other Autoimmune Disorders. Diabetes Case Rep 2016; 1:114. doi: 10.4172/2572-5629.1000114.
- Matz H, Tur E. Vitiligo. Curr Probl Dermatol 2007; 35: 78-102.
- Kakourou T, Kanaka-Gantenbein C, Papadopoulou A, Kaloumenou E, Chrousos GP. Increased prevalence of chronic autoimmune (Hashimoto's) thyroiditis in children and adolescents with vitiligo.Am Acad Dermatol. 2005;53(2):220-3.
- Spritz RA. The genetics of generalized vitiligo. Curr Dir Autoimmun 2008;10: 244-257.
- Rashtak S, Pittelkow MR. Skin involvement in systemic autoimmune diseases. Curr Dir Autoimmun 2008; 10: 344-358.
- Oiso NT, Fukai K, Kabashima K, Kawada A, Suzuki T. Generalized vitiligo and associated autoimmune diseases in japanese patients and their families. AllergolInt 2011;60(4): 505 -508.
- Raveendra L, Hemavathi RN, Rajgopal S. A Study of Vitiligo in Type 2 Diabetic Patients. Indian J Dermatol. 2017 Mar-Apr;62(2):168-170. doi: 10.4103/ijd.IJD_360_16.
- Abbot Alison. Scientists bust myth that our bodies have more bacteria than human cells; Decades-old assumption about microbiota revisited. Nature doi:10.1038/nature2016;19136
- Schnorr, S. L. et al. Gut microbiome of the Hadza hunter-gatherers. Nat. Commun 2014; 5:3654 doi: 10.1038/ncomms4654.
- Rampelli, S., Schnorr, S. L., Consolandi, C., Turroni, S., Severgnini, M., Peano, C., et al. Metagenome sequencing of the Hadza hunter-gatherer gut microbiota. Curr. Biol 2015; 25, 1682–1693. doi: 10.1016/j.cub.2015.04.055
- Bhagyanathan Meera, Deepak Dhayanithy, Vijayan Ampaya Parambath, and R. Bijayraj. Acanthosis nigricans: A screening test for insulin resistance – An important risk factor for diabetes mellitus type-2. J Family Med Prim Care. 2017 Jan-Mar; 6(1): 43–46. doi: 10.4103/2249-4863.214961
- Brickman WJ, Binns HJ, Jovanovic BD, Kolesky S, Mancini AJ, Metzger BE. Acanthosis Nigricans: A Common Finding in Overweight Youth. Paediatric Dermatology 2007;Vol. 24 No. 6 601–606.
- Vijayan A. P., Varma K. K., Meera Bhagyanathan, Dinesh K. B., Divia Nath K. R. and Bijayraj R. Three physical markers of insulin resistance (body mass index, waist circumference and acanthosis nigricans): A cross-sectional study among children in south India. Medical Practice and Review 2011; Vol. 2(4), pp. 37-43.
- Meghana Madhukar Phiske. An approach to acanthosis nigricans. Indian Dermatol Online J. 2014 Jul-Sep; 5(3): 239–249. doi: 10.4103/2229-5178.137765 PMCID: PMC4144206
- Schwartz RA. Acanthosis nigricans. J Am Acad Dermatol. 1994; 31:1–19.
- Matsuoka LY, Wortsman J, Gavin JR, Goldman J. Spectrum of endocrine abnormalities associated with acanthosis nigricans. Am J Med. 1987;83(4):719-25.
- Habif TP. Cutaneous manifestations of internal disease. In: Clinical Dermatology: A Colour Guide to Diagnosis and Therapy. 5th ed. Edinburgh, U.K.; New York, N.Y.: Mosby Elsevier; 2010. http://www.clinicalkey.com.
- Burke JP, Duggirala R, Hale DE, Blangero J, Stern MP: Genetic basis of acanthosis nigricans in Mexican Americans and its association with phenotypes related to type 2 diabetes. Hum Genet 2000; 106:467–472.
- Braunstein I. Acanthosis nigricans. In: Callen J, Ofori AO. UpToDate. Waltham, MA: UpToDate; March, 2014; http://www.uptodate.com/contents/acanthosis-nigricans
- Hamman R.Genetic and environmental determinants of non-insulin dependent diabetes mellitus (NIDDM). Diabetes MetabRev1992; 8:287–338 10.
- Kahn C. Insulin action, diabeto-genes, and the cause of type II diabetes. Diabetes 1994; 43:1066–1084
- Bouchard C Genetics and the metabolic syndrome. Int J Obesity 1995 ;19[Suppl]:S52–S59 12.
- Yki-Ja¨rvinen H Role of insulin resistance in the pathogenesis of NIDDM. Diabetologia1995; 38:1378–1388
- Hales C, Barker DM Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia 1992; 35:595– 601 14
- Karam JH, Grodsky GM, Forsham PH Excessive insulin response to glucose in obese subjects as measured by immuno- chemical assay. Diabetes1963; 12:197-204
- C. N. Hales 1 and D. J. P. Barker Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia 1992; 35:595~601.
- American Diabetes Association: Type 2 diabetes in children and adolescents (Consensus Statement). Diabetes Care 2000; 23:381–389.
- Richards GE, Cavallo A, Meyer WJ, Prince MJ, Peters EJ, Stuart CA, Smith ER: Obesity, acanthosis nigricans, insulin resistance and hyperandrogenemia: paediatric perspective and natural history. J Pediatr 1985; 107:893–897,
- Alberta S. Kong, Robert L. Williams, Melissa Smith, Andrew L. Sussman, Betty Skipper, Andrew C, Robert L. Rhyne, Acanthosis Nigricans and Diabetes Risk Factors: Prevalence in Young Persons Seen in Southwestern US Primary Care Practices Ann Fam Med. 2007; 5(3): 202–208. doi: 10.1370/afm.678
- Yamazaki Ito S, Yoshida H. Acanthosis nigricans is a reliable cutaneous marker of insulin resistence in obese Japanese children. Paeditr Int. 2003; 45:701–705. doi: 10.1111/j.1442-200X.2003.01812.
- Grandhe NP, Bhansali A, Dogra S, Kumar B. Acanthosis nigricans: relation with type 2 diabetes mellitus, antropometric varables, and body mass in Indians. Post Med J. 2005; 81:541–544. doi: 10.1136/pgmj.2004.028308.
- Phiske MM. An approach to acanthosis nigricans. Indian Dermatol Online J. 2014;5(3):239-49.
- Cordain L, Eades MR, Eades MD. Hyperinsulinemic diseases of civilization: more than just Syndrome X. Comparative Biochemistry and Physiology. Part A, Molecular and Integrative Physiology 2003;136, 95–112.
- Pandarakalam James Paul. Is autoimmunity involved in the aetiology of schizophrenia? Prog. Neurol. Psychiatry, 2013;17: 24–28. doi: 10.1002/pnp.267
- Pandarakalam James Paul. The Autoimmune and Infectious Etiological Factors of a Subset of Schizophrenia. BJMP 2015;8(4): a831.
- Martin FI, Alford FP. Insulin sensitivity in schizophrenia. Br Med J 1970; 2:50.
- Allison, D. B. et al. The distribution of body mass index among individuals with and without schizophrenia. The Journal of clinical psychiatry.1999; 60: 215–20.
- Manu, Peter; Al-Dhaher, Zainab, MSc; Dargani, Navin ; Correll, Christoph U. Acanthosis Nigricans During Treatment With Aripiprazole. American Journal of Therapeutics. 2014; 21(3):90–93.
- Kapoor S. “Diagnosis and treatment of acanthosis nigricans.” Skinmed. 2010;8(3):161-4; quiz 165.
- Goldsmith LA, et al., eds. Cutaneous manifestations of internal malignant disease. In: Fitzpatrick's Dermatology in General Medicine. 8th ed. New York, N.Y.: The McGraw-Hill Companies; 2012. http://www.accessmedicine.com.
- World Health Organization, Global Report on Diabetes. Geneva, 2016. Accessed 30 August 2016.
- "Simple treatment to curb diabetes". January 20, 2014. Archived from the original on 2014-02-02.
- Wild S, Roglic G, Green A, Sicree R, King H. "Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030". Diabetes Care 2004; 27 (5): 1047–53. doi:10.2337/diacare.27.5.1047. PMID 15111519.

The above article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.




