Ketamine and Propofol Combination Used in Deep Sedation Reduces Opioid Use and Adverse Outcomes in Pediatric Esophagogastroduodenoscopy in Ages 2-18 Years
David C Mari & Abhik K Biswas
Cite this article as: BJMP 2019;12(3):a020
|
|
Abstract Background: EGD procedures can elicit significant pain and cause complications in children. Therefore it is essential to use sedation agent(s) that reduce pain and are safe. Abbreviations: Esophogastroduodenoscopy (EGD), length of stay (LOS), mean arterial blood pressure (MAP), heart rate (HR), respiratory rate (RR), and Children’s Hospital of Eastern Ontario Pain Scale (CHEOPS). |
Introduction:
Aesophagogastroduodenoscopy (EGD), is a common same-day procedure used for both diagnostic and therapeutic purposes during which a small flexible fibreoptic tubular camera is introduced through the mouth and advanced through the pharynx into the oaesophagus, stomach, and duodenum. EGD procedures are performed under deep sedation, since they can elicit significant pain, discomfort, and anxiety. When pain is not controlled properly, this can lead to an increase use of adjunctive medications such as opioids. This can extend the length of stay and increase adverse outcomes.1 In a prospective, randomised, double-blinded study, Bedirli et al. found that use of opioid medications such as fentanyl are associated with increased adverse outcomes.2
Since procedural sedation-related complications (such as hypoxia, hypotension, desaturation, and emergent airway intervention) remain one of the biggest challenges in EGD procedures, it is important to select the correct medications to reduce these complications.3 Currently, propofol is the most common and popular main procedural sedation agent used for EGD procedures.4,5
Propofol is the preferred main intravenous agent in EGD procedures due to its amnestic, sedative, and hypotonic properties.6 It has also been favoured due to its ultra-rapid response and duration of effects, usually taking 30-60 seconds for onset of action and lasting up to 4-8 minutes.7 However, propofol has been associated with significant adverse effects that include dose-related hypotension, bradycardia, laryngospasms, and apnoea.8,9 Propofol does not have analgesic properties and will usually be combined with opioids for pain control.10
Ketamine is classified as a dissociative anaesthetic and is known to provide analgesia and amnesia. Important to note, it causes less respiratory or cardiovascular depression when used alone for children greater than 4 months of age.11 However, ketamine alone can cause such side effects of laryngospasm, increase secretions, and vomiting.12,13
The mixture of propofol and ketamine in one syringe (coined ketofol) has been shown to be effective in sedation for various procedures, such as spinal anaesthesia,14 along with orthopaedic15 and cardiovascular procedures16 in adults and children. Use of this combination has been favoured in brief but painful emergency room procedures due to the opposing haemodynamic and respiratory effects of both sedative medications.17 The negative cardiac effects produced by propofol can be attenuated with the use of ketamine, resulting in an increase in mean arterial pressures and cardiac indices.18,19 The complementary effect of both mediations has enabled the use of lower doses for each medication, thus lowering the toxicity and side effects.20,21 Although there are studies comparing activities of ketofol sedations,22-25 there has not been a study published on ketofol compared to propofol use in children during EGD procedures.
The primary goal of this study was to determine if there were differences in the outcomes of adverse cardiac and pulmonary events, vital sign parameters including objective pain, and administration of adjunct pain medication (for which fentanyl was used in this study) during EGD procedures between propofol and ketofol groups. A secondary goal was to determine if there was a difference in site performance metrics for EGD procedures (sedation time, stop of sedation to discharge time, and length of stay) between propofol and ketofol groups.
Methods:
This study protocol was approved by the Naval Medical Center Portsmouth Institutional Review Board in compliance with all applicable federal regulations governing the protection of human subjects. Research data was derived from an approved IRB protocol: number NMCP.2018.0021. Written informed consent was not required by the Naval Medical Center Portsmouth IRB, as this data concerned historical dae-identified patients.
Study Design
This was a single centre, retrospective study of a cohort of children (ranging from 2 to 18 years). Data was collected from March 2011 to September 2013 at Naval Medical Center Portsmouth’s Paediatric Sedation Centre. EGD and sedation protocol was not changed during this time period. EGD procedures were performed by the same paediatric gastroenterologists throughout this time period. Sedation was performed by the same paediatric intensivists throughout this time period. All patients used in this study were involved in a same day EGD procedure. Forty-one patients underwent deep sedation via propofol as main sedation agent from March 2011 to May 2012. Forty-nine patients underwent deep sedation via propofol and ketamine combination as main sedation agent from May 2012 to September 2013. Each main sedation agent was given in a similar fashion as an initial intravenous loading dose based on 1 mg/kg propofol followed by an intravenous infusion of 200-250 mcg/kg/minute that was started less than 10 minutes prior to start of the procedure and stopped at the end of the procedure. Ketofol solution used was a 1:5 ratio of ketamine to propofol (40 mg:200 mg). One mcg/kg of fentanyl was given when the sedationist during the EGD perceived the patient experiencing pain. Exclusion criteria included: patients less than 2 years and greater than 18 years, those less than 10 kg, and patients receiving adjunct medications other than fentanyl, ondansetron, or lidocaine.
Data Collection
Fourteen patients were excluded due to weight less than or equal to 10 kg. In addition, 12 patients were excluded due to age less than 2 years or greater than 18 years. Past medical history, ASA class, procedure indications, age, weight, sex, pain scores, vital signs (mean arterial blood pressure [MAP], heart rate [HR], and respiratory rate [RR]), unplanned events (hypoxia defined as SPO2 less than 85% at any time point, and hypotension defined as mean arterial blood pressure with greater than 20% decrease from baseline blood pressure), emergent airway intervention (defined as apnoea needing bag mask ventilation or CPAP use), and unplanned intubation. Vitals and pain scores were obtained from initial presentation in the sedation suite, start of procedure, every 5 minutes during the procedure to stop of sedation, and at time of discharge. Midway procedure vitals used for statistical analysis were obtained by selecting vitals that were at the half-way point of the patient’s EGD procedure. The Children’s Hospital of Eastern Ontario Pain Scale (CHEOPS) was performed every 5 minutes during the EGD procedure by an independent United States Navy Corpsman and used to evaluate patient’s pain prior, during, at stop of sedation, and after procedure for this study.26
Statistics
All statistics performed in this study were calculated using SPSS Statistics programme (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp.). Mann-Whitney U nonparametric test between independent groups was performed to compare age, weight, total EGD procedure time, total time of sedation during EGD procedure, time from stop of sedation to discharge, total length of stay, and total fentanyl use (in mcg/kg) between the two main groups in this study (propofol and ketofol group). The significance level was set to 0.008 after a Bonferroni was corrected, in order to address the probability of making one or more false discoveries when performing multiple hypotheses tests. A repeated measures ANOVA was run to determine the effect of treatments over time for HR, MAP, and RR. Mauchly's test of sphericity was performed for both age groups. The Fisher Exact test was performed to compare propofol to ketofol in unplanned hypotensive events and unplanned apnoea requiring bag valve mask or CPAP. Pearson Correlation statistics were performed to study possible correlations between propofol or fentanyl and sedation time or length of stay. Statistical significance for Pearson Correlation statistics was considered when two-tailed p<0.001.
Results:
Table 1. Demographic divided into 2 age groups. EE= eosinophilic esophagitis. * indicates statistically significant difference.
|
Sedation Risk Age Groups |
|||||||||
|
2-11 years old |
12-18 years old |
||||||||
|
Propofol n=26 |
% |
Ketofol n=27 |
% |
Propofol n=15 |
% |
Ketofol n=22 |
% |
||
|
General Demographics |
Male |
11 |
42.3% |
16 |
59.3% |
6 |
40% |
10 |
45.5% |
|
Female |
15 |
57.7% |
11 |
40.7% |
9 |
60% |
12 |
54.5% |
|
|
Mean Age (years old) |
6 |
7 |
15 |
16 |
|||||
|
Mean Weight (kg) |
21.6 |
23.7 |
57.7 |
57.4 |
|||||
|
ASA I, II |
26 |
100% |
25 |
92.6% |
15 |
100% |
22 |
100% |
|
|
ASA III |
0 |
0% |
2 |
7.4% |
0 |
0 |
|||
|
EGD Indications |
EE |
4 |
15.4% |
4 |
14.8% |
2 |
13.3% |
0 |
0% |
| GERD |
11 |
42.3% |
9 |
33.3% |
2 |
13.3% |
5 |
22.7% |
|
| Dysphagia or Feeding Intolerance |
0 |
0% |
3 |
11.1% |
4 |
26.6% |
4 |
18.1% |
|
| Foreign Body Ingestion |
1 |
3.8% |
2 |
7.4% |
0 |
0% |
0 |
0% |
|
| Failure to Thrive |
3 |
11.5% |
1 |
3.7% |
0 |
0% |
0 |
0% |
|
| Abdominal Pain |
7 |
26.9% |
9 |
33.3% |
6 |
40% |
11 |
50% |
|
| Recurrent Emesis |
3 |
11.5% |
1 |
3.7% |
1 |
6.6% |
1 |
4.5% |
|
| Gastritis |
0 |
0% |
0 |
0% |
1 |
6.6% |
3 |
13.6% |
|
| Other |
2 |
7.7% |
0 |
0% |
2 |
13.3% |
4 |
18.1% |
|
|
Unplanned Events |
Hypotension (blood pressure >20% decrease from baseline) |
4 |
15.4% |
1 |
3.7% |
2 |
13.3% |
0 |
0% |
| Apnea requiring bag valve mask or CPAP |
14 |
53.8%* |
1 |
3.7%* |
5 |
33.3%* |
0 |
0%* |
|
| Intubations |
0 |
0% |
0 |
0% |
1 |
6.6% |
0 |
0% |
|
Figure 1. Propofol amount total per weight (mg/kg). Bars represent standard error of the mean. There was no significant difference between the propofol and ketofol in all age groups.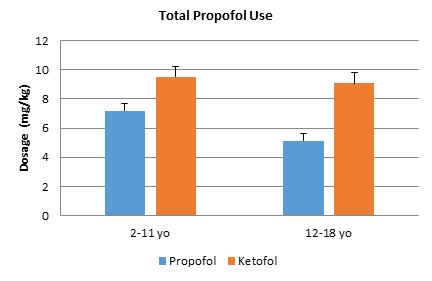
Figure 2. Fentanyl amount total per weight (mcg/kg). Bars represent standard error of the mean. In all age groups, there was a significant difference between propofol and ketofol. *p<.008.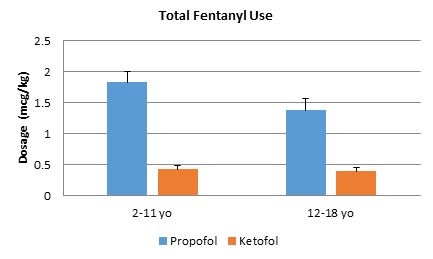
Figure 3. Vital signs. Mean arterial pressures for ages A) 2-11 years and B) 12-18 years. Main sedative agents’ MAPs were statistically different for ages 2-11 years (p =0.004), but not for ages 12-18 years (p =0.224) for all time periods. Heart rate for ages C) 2-11 years and D) 12-18 years. For Heart Rate, there was a statistically significant interaction between treatment and time for both age group using the Greenhouse-Geisser correction, p =0.002, p =0.014. Main sedative agents’ HRs showed to be statistically different for both age groups, p <0.001 and p =0.004.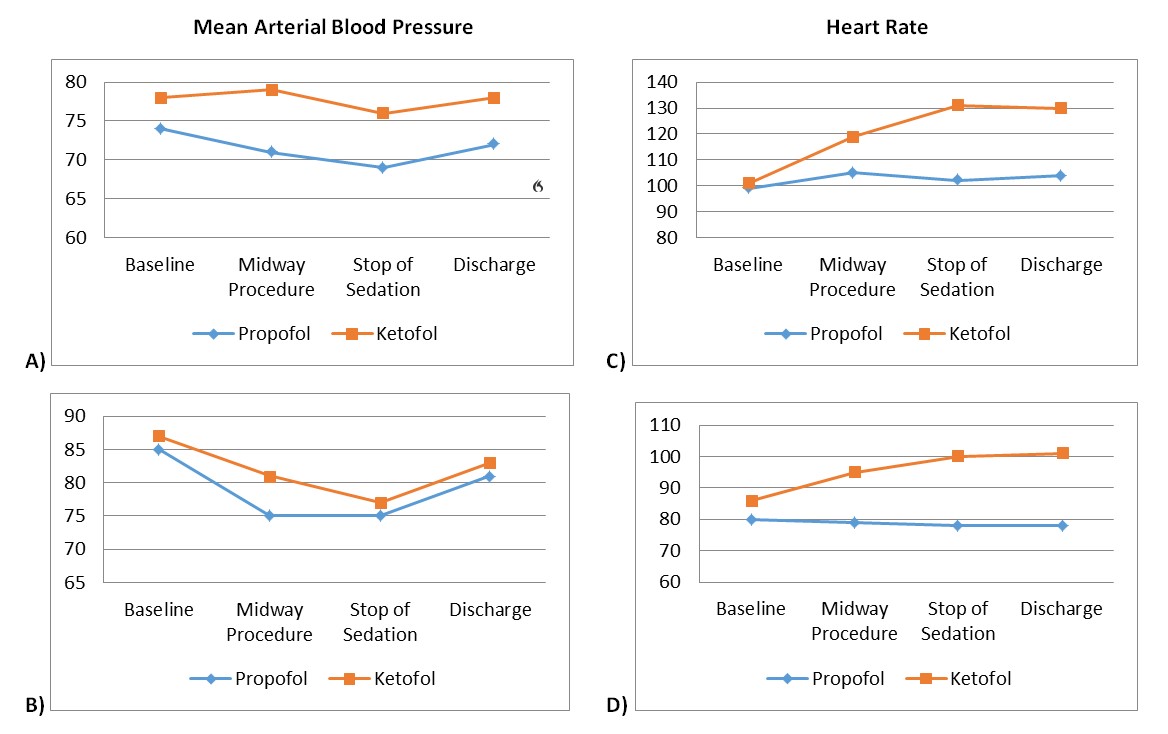
Figure 4. Duration of time. A) total sedation time, B) stop of sedation to discharge, and C) total length of stay. In all age groups, there was no significant difference between propofol and ketofol. Bars represent standard error of the mean. *p<0.008.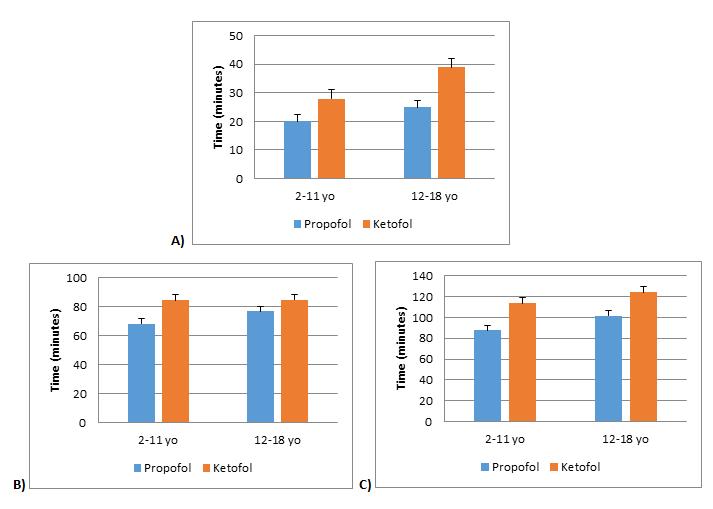
Figure 5. Pearson Correlation. Propofol versus total sedation time for ages A) 2-11 years (p<0.01*), and B) 12-18 years (p<0.01*). Propofol versus length of stay for ages C) 2-11 years (p<0.01*), and D) 12-18 years (p<0.01*). * Correlation is significant at the 0.01 level (2-tailed).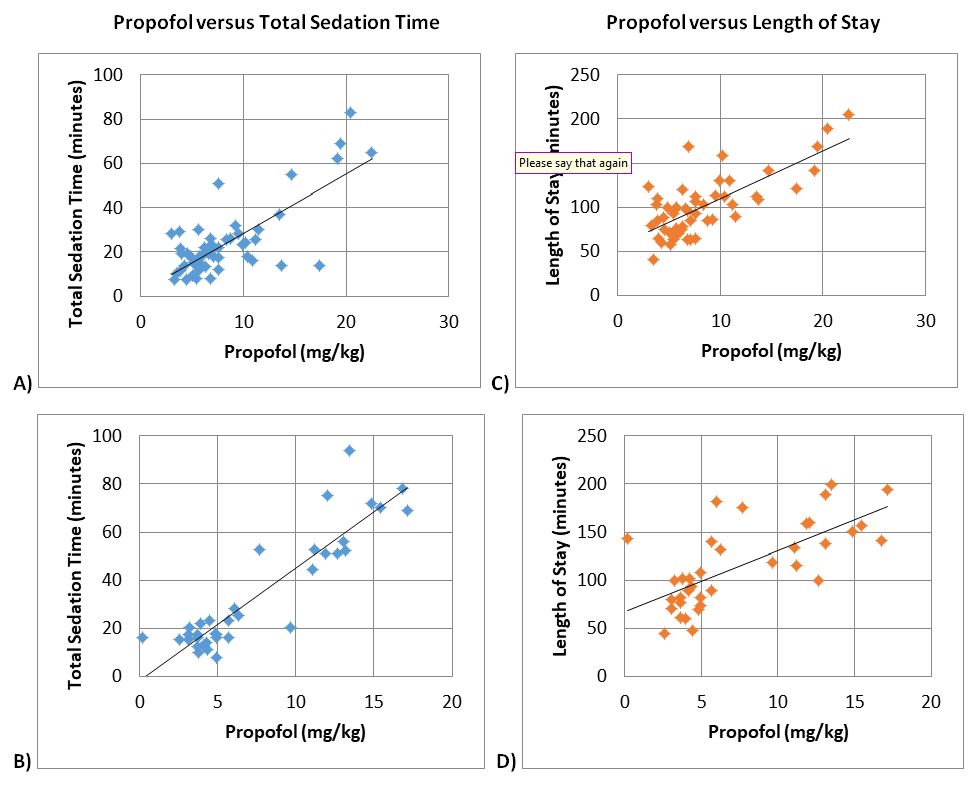
Figure 6. Pearson Correlation. Fentanyl versus total sedation time for ages A) 2-11 years (p=0.506), and B) 12-18 years (p=0.961). Fentanyl versus length of stay for ages C) 2-11 years (p=0.378), and D) 12-18 years (p=0.352). No significant correlation was not seen for all age groups.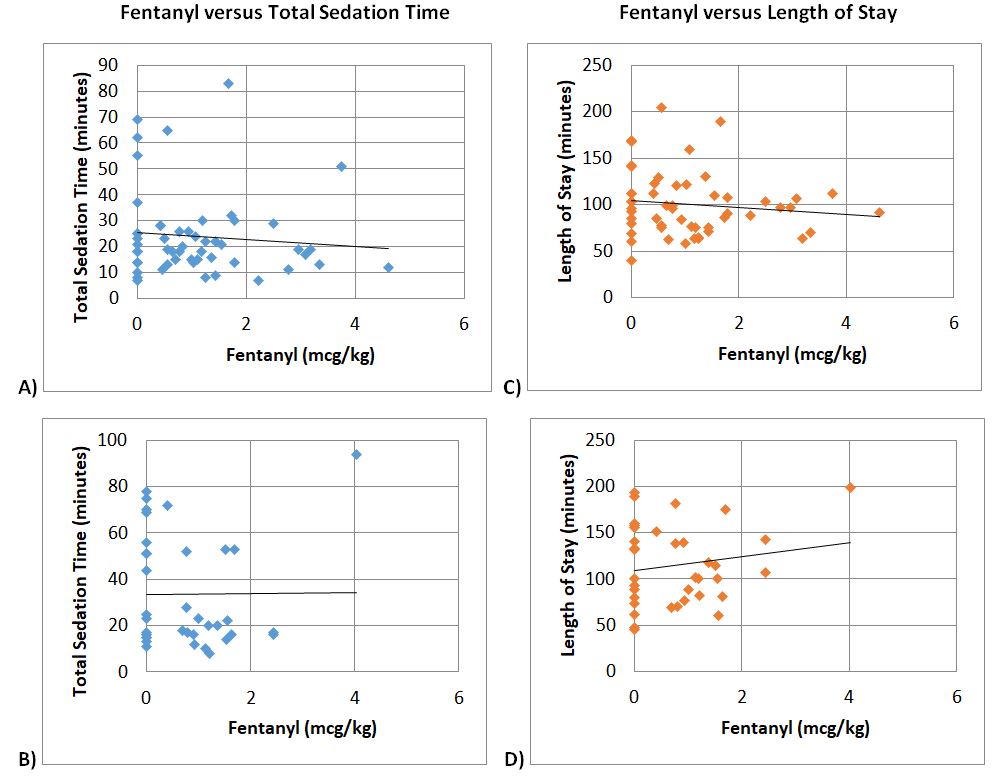
Ninety patients were retrospectively analysed in this study. Baseline demographics were similar between the groups, except for gender proportions (Table 1). Similar amounts of propofol per weight were used in each group (Figure 1). Ketofol significantly reduced fentanyl use in all age groups by ≥.99 mcg/kg compared to propofol alone (p<0.008 for all age groups, Figure 2). In the 2-11 year old age group, the propofol group required a mean fentanyl dose of 1.96 mcg/kg ± 1.24 mcg/kg and the ketofol group required a mean fentanyl dose of 0.44 mcg/kg ± 0.49 mcg/kg; thus ketofol required about 1.52 mcg/kg less fentanyl during EGD procedures. In the 12-18 year old group, the propofol group required a mean fentanyl dose of 1.38 mcg/kg ± 1.05 mcg/kg and the ketofol group required a mean fentanyl dose of 0.39 mcg/kg ± 0.59 mcg/kg; thus ketofol required about 1 mcg/kg less fentanyl during EGD procedures.
Vital signs (HR, RR, and MAP) and CHEOPS pain scores were obtained and analysed for baseline vitals, at the procedure midpoint, at stop of sedation, and at time of discharge. CHEOPS pain scores’ mean was 6 throughout all time periods for both groups, thus there was no statistically significant difference.
A repeated measures ANOVA was run to determine the effect of treatments over time for RR. There was sphericity for the interaction term, as assessed by Mauchly's test of sphericity in both age groups (p =0.070, p =0.762). For RR, there was not a statistically significant interaction between treatment and time for both age groups, p =0.163, p =0.804. The treatments were not found to be statistically different for either age group, p =0.736 and p =0.224.
A repeated measures ANOVA was run to determine the effect of treatments over time for HR. As assessed by Mauchly's test of sphericity, no sphericity was found in either age group (p <0.05). For HR, using the Greenhouse-Geisser correction, a statistically significant interaction was found between treatment and time for both age group, p =0.002, p =0.014. The treatments were found to be statistically different for both age groups, p <0.001 and p =0.004. A repeated measures ANOVA was run to determine the effect of treatments over time for MAP. Sphericity was found in both age groups for the interaction term, as assessed by Mauchly's test of sphericity (p =0.209, p =0.269). For MAP, there was not a statistically significant interaction between treatment and time for either age group, p =0.261, p =0.591. The treatments were statistically different for the 2-11 year old group (p=0.004), but not statistically different for the 12-18 year old group (p=0.224). (Figure 3 A-D)
Unplanned events were analysed for clinically significant hypoxia, hypotension, emergent airway intervention, and unplanned intubations. Ketofol compared to propofol had fewer hypotensive events in the 2-11 year old age group (3.7% versus 15.4%, p=0.192) and in the 12-18 year old age group (0% versus 13.3%, p=0.144). Ketofol compared to propofol had statistically significant fewer apnoea events requiring bag valve mask or CPAP intervention for the 2-11 year old group (3.7% versus 53.8%, p<0.001) and for the 12-18 year old group (0% versus 33.3%, p=0.005). There were no significant hypoxia events. There was one unplanned intubation in the propofol group of a healthy ASA I twelve year old female who had a 20 mL emesis episode after a loading dose of propofol was given on induction. (Table 1)
With propofol leading to significantly more fentanyl usage, more hypotension and emergent airway intervention during EGD procedures, we performed analysis to see if there was an effect on sedation time, time from stop of sedation to discharge, and length of stay (LOS). In all age groups, there was no statistical difference between propofol and ketofol for sedation time (p=0.115 for ages 2-11 years, p=0.124 for ages 12-18 years), time from stop of sedation to discharge (p=0.033 for ages 2-11 years, p=0.511 for ages 12-18 years), and LOS (p=0.026 for ages 2-11 years, p=0.109 for ages 12-18 years) (Figure 4). Based on Pearson correlation test, it was established that propofol was positively correlated with sedation time and LOS (+0.753 for sedation time and +0.611 correlation for length of stay with <0.001 significance) (Figure 5). There was no significant correlation between fentanyl and sedation time or LOS (Figure 6).
Discussion:
This was a retrospective study looking at 90 paediatric patients, ages 2-18 years, undergoing EGD procedures with either propofol or ketofol as the main sedative medication. Patients in this study had similar weights, ages, ASA scores, and EGD indications; however there baseline demographics were not similar with regards to gender proportion (Table 1). Based on the large prospective database on sedation use for procedures outside the operating room obtained by the Paediatric Sedation Research Consortium,27 we separated our patient population into two age risk groups to allow our results to be generalisable. To our knowledge, this study is the first to show that ketofol, when compared to propofol, significantly reduces fentanyl use (≥.99 mcg/kg, Figure 2) and cardiopulmonary adverse outcomes (Table 1) in paediatric EGD procedures.
EGD procedures are known to be invasive and painful. To decrease the pain appreciated by a patient, various adjuncts are often utilised. In our study, we used fentanyl for adjunct pain control. In both main sedation medication groups (propofol and ketofol), there was no statistical significance seen in objective pain based on CHEOPS scores with a mean score of 6 in all age groups (p>0.05). However, the amount of adjunct needed to maintain adequate pain control between both groups statistically and clinically differed (Figure 2). In all age groups, the propofol group compared to the ketofol group required almost 1 mcg/kg more of fentanyl, which leads to the potential for the patient to experience higher incidence of side effects, such as respiratory depression and hypoxia.28-30
The mechanism that lead to this significant difference in opioid demand between ketofol and propofol is most likely due to ketamine’s ability to stimulate opioid sigma receptors,31 thus leading to opioid sparing effects. It is unclear if propofol and ketamine interaction heightens this opioid sparing effect, because ketamine alone has not been shown to lead to opioid sparing effects in children.32
In a review of our population’s vital signs, there was a significantly higher MAP in the ketofol group compared to the propofol group for ages 2 to 18 years (Figure 3 A-B). In addition, the ketofol group had statistically higher HR compared to the propofol group for ages 2 to 18 years (Figure 3 C-D). It is our thought that this increase in MAP and HR in the ketofol group mediated by ketamine is a protective factor against major hypotensive changes (31). It is this effect that minimised unplanned hypotensive events in our ketofol group compared to the propofol group by 11.7% in ages 2-11 years and 13.3% in ages 12-18 years (Table 1).
One of the more noticeable differences in our main sedative medication groups was the unplanned apnoea events requiring CPAP or bag mask ventilation intervention. In our 2 to 11 year age group, propofol had 50.1% more apnoea events needing intervention compared to ketofol. In our 12 to 18 year old group, propofol had 33.3% more apnoea events needing respiratory intervention compared to ketofol. This was found to be statistically significant. It is unclear if this is also clinically significant since no emergent airway intubation was needed for these events. However, there was 1 emergent airway intubation in the 12-18 year old propofol group. Based on the review of the medical records, it appears that these apnoea events needing respiratory intervention were mostly associated after the initial loading dose of either propofol or ketofol prior to or at the start of the infusion. Thus, bring into question if not starting with a loading dose bolus would decrease adverse effects.
Despite the propofol group requiring more respiratory intervention, increased fentanyl use, and less haemodynamic stability seen, there was no statistical differences in total sedation time and LOS between the two main sedation medication groups (Figure 4). Yet when we ran correlation statistics, there was a positive correlation to increased propofol dosages, total sedation time, and LOS in ages 2 to 18 years (Figure 5); therefore, if our procedures were longer, there could be a statistically and possibly clinically significant increase in total sedation time and LOS for the propofol only group. Thus, we can infer that propofol compared to ketofol is not the main sedation medication of choice for longer similarly painful procedures in children ages 2 to 18 years, such as EGD procedure followed by a colonoscopy.
A limitation of our study is that it is retrospective. With that, we were not able to blind our sedationists to the main sedation medication that was chosen and could not control the interventions that were performed. Yet, based on strict exclusion criteria, we were able to control the interventions used in our analysis. Also, in our study we did not analyse patients under the age of 2 years. Reviewing the data, we had seven patients, and thus this patient population size was not powered to perform the proper statistical analysis. Another limitation to consider is the time difference in procedure protocols. However, since this study included over 2 years of data, procedure protocols were the same. Additionally, the EGD indications were similar between ketofol and propofol (Table 1). Another consideration of this study is generalisability. This study was conducted at a single centre, but the way we analysed our results makes it easier to perform large scale prospective studies to further investigate our findings. Last limitation is directly correlating fentanyl dose with a significant adverse event. Due to the short length of the EGD procedure, constant sedation medication infusion, and fentanyl dose, it is difficult to directly say that a particular fentanyl dose alone leads to an adverse event. Therefore we can only speculate based on correlation.
Conclusions:
Our study found that ketofol significantly reduced fentanyl use in all age groups by ≥0.99 mcg/kg and cardiopulmonary adverse events compared to propofol alone. In addition, an increase in the amount of propofol was positively correlated to increasing LOS and total sedation time for a child undergoing an EGD procedure. This suggests that increasing amounts of propofol leads to greater LOS. To conclude, our study indicates that ketofol can be a safer alternative to propofol use alone for deep sedation in EGD procedures for children ages 2 to 18 years.
|
Acknowledgements We like to thank all Paediatric Intensivists and Paediatric Gastroenterologists involved in patient care. We would also like to thank Andrea McGlynn with her help and guidance with statistics used in this study. Authors in this manuscript have no conflicts of interest to disclose. This study was completed with no external or internal funding. We are military service members and employees of the U.S. Government. This work was prepared as part of our official duties. Title 17 U.S.C. 105 provides that “Copyright protection under this title is not available for any work of the United States Government.” Title 17 U.S.C. 101 defines a United States Government work as a work prepared by a military service member or employee of the United States Government as part of that person’s official duties. The views expressed in this manuscript reflect the results of research conducted by the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defence, or the United States Government. Competing Interests None declared Author Details DAVID C. MARI, D.O., Capt USAF;Division of Pediatric Medicine, Naval Medical Center Portsmouth, 620 John Paul Jones Circle, Portsmouth, VA 23708. ABHIK K. BISWAS, M.D., CAPT (ret) USN; Division of Pediatric Critical Care Medicine, Naval Medical Center Portsmouth, 620 John Paul Jones Circle, Portsmouth, VA 23708. CORRESPONDENCE: DAVID C. MARI, Division of Pediatric Medicine, Naval Medical Center Portsmouth 620 John Paul Jones Circle, Portsmouth, VA 23708. Email: david.c.mari@gmail.com |
References
- Samer Ammar M, Pfefferkorn MD, Croffie JM, et al. Complications after outpatient upper GI endoscopy in children: 30-day follow-up. Am J Gastroenterol. 2003; 98: pp. 1508-1511.
- Bedirli N, Egritas O, Cosarcan K, et al. A comparison of fentanyl with tramadol during propofol-based deep sedation for pediatric upper endoscopy. Paediatr Anaesth. 2012 Feb; 22(2): 150-155.
- Orel R, Brecelj J, Dias JA, et al. Review on sedation for gastrointestinal tract endoscopy in children by non-anesthesiologists. World J Gastrointest Endosc. 2015 Jul 25; 7(9): 895-911.
- Mustafaeva MN, Mizikov VM, and Kochneva ZV. Drug sedation during digestive tract endoscopy: current trends. Anesteziol Reanimatol 2009; 4: 32-38.
- Kochlar GS, Gill A, and Vargo JJ. On the Horizon: The Future of Procedural Sedation. Gastrointestinal Endoscopy Clin N Am. 2016; 26: 577-592.
- Watcha MF, Simeon RM, White PF, et al. Effect of propofol on the incidence of postoperative vomiting after strabismus surgery in pediatric outpatients. Anesthesiology, 1991, pp 2049.
- McCollum JS, Milligan RK, and Dundee JW. The antiemetic action of propofol. Anaesthesia. 1988; 43: 239-240.
- Hig Jr CC, McLeskey CH, Nahrwold ML, et al. Hemodynamic effects of propofol: data from over 25,000 patients. Anesth Analg, 1993, pp S21-S29.
- Claeys MA, Gepts E, and Camu F. Haemodynamic changes during anaesthesia induced and maintained with propofol. Br J Anaesth, 1988, pp 3-9.
- Varddi A, Salem Y, and Padeh S. Is propofol safe for procedural sedation in children? A prospective evaluation of propofol versus ketamine in pediatric critical care. Crit Care Med, 2002, pp 1231-1236.
- 11. Grunwell JR, Travers C, McCracken CE, et al. Procedural Sedation Outside of the Operating Room Using Ketamine in 22,645 Children: A Report From the Pediatric Sedation Research Consortium. Pediatr Crit Care Med. 2016 Dec; 17(12): 1109-1116.
- 12. Kurdi MS, Theerth KA, and Deva RS. Ketamine: Current applications in anesthesia, pain, and critical care. Anesth Essays Res. 2014 Sep-Dec; 8(3):283-290.
- Green SM, Roback MG, and Krauss B. Laryngospasm during emergency department ketamine sedation: A case-control study. Pediatr Emerg Care. 2010;26:798–802.
- Frizelle HP, Duranteau J, Samii K. A comparison of propofol with a propofol-ketamine combination for sedation during spinal anesthesia. Anesth Analg, 1997, pp 1318-1322.
- Weatherall A and Venclovas R. Experience with a propofol-ketamine mixture for sedation during pediatric orthopedic surgery. Paediatr Anaesth 2010; 20: 1009-1016.
- Mourad M, El-Hamamsy M, Anwar M, et al. Low dose ketamine reduces sedative doses of propofol during ambulatory trans-oesophageal echocardiography. Egyptian Journal of Anaesthesia, 2004, pp 41-46.
- Mortero RF, Clark LD, Tolan MM, et al. The effects of small-dose ketamine on propofol sedation: respiration, postoperative mood, perception, cognition, and pain. Anesth Analg, 2001, pp 1465-1469.
- Willman EV and Andolfatto G. Prospective evaluation of “ketofol”(ketamine/propofol combination) for procedural sedation and analgesia in the emergency department. Ann Emerg Med 2007; 49: 23-30.
- 19. Kochs E, Scharein E, Mollenberg O, et al. Analgesic efficacy of low dose ketamine. Somatosensory-evoked responses in relation to subjective pain ratings. Anesthesiology 1996; 85: 304–314.
- Badrinath S, Avramov MN, Shadrick M, et al. The use of a ketamine-propofol combination during monitored anesthesia care. Anesth Analg 2000; 90: 858-862.
- Loh G and Dalen D. Low-dose ketamine in addition to propofol for procedural sedation and analgesia in the emergency department. Ann Pharmacother 2007; 41:485-492.
- Andolfatto G, Abu-Laban RB, Zed PJ, et al. Ketamine-propofol combination (ketofol) versus propofol alone for emergency department procedural sedation and analgesia: a randomized double-blind trial. Ann Emerg Med 2012; 59: 504-512.
- Sakai T, Singh H, Mi WD, et al. The effect of ketamine on clinical endpoints of hypnosis and EEG variables during propofol infusion. Acta Anaesthesiol Scand 1999; 43: 212–216.
- Hui TW, Short TG, Hong W, et al.Additive interactions between propofol and ketamine when used for anesthesia induction in female patients. Anesthesiology 1995; 82: 641–648.
- Badrinath S, Avramov MN, Shadrick M, et al.Use of ketamine–propofol admixture during monitored anesthesia care. Anesthesiology 1997; 87: A10.
- McGrath PJ, Johnson G, Goodman JT, et al. CHEOPS: a behavioral scale for rating postoperative pain in children. In: H.L. Fields, R. Dubner and F. Cervero (Eds.), Advances in Pain Research and Therapy, Raven Press, New York, 1985, pp 395-402.
- Cravero JP, Blike GT, Beach M, et al. Incidence and nature of adverse events during pediatric sedation/anesthesia for procedures outside the operating room: report from the Pediatric Sedation Research Consortium. Pediatrics. 2006 Sep; 118(3): 1087-1096.
- Godambe SA, Elliot V, Matheny D, et al. Comparison of propofol/fentanyl versus ketamine/midazolam for brief orthopedic procedural sedation in a paediatric emergency department. Pediatrics 2003; 112: 116–123.
- Skokan EG, Pribble C, Bassett KE, et al. Use of propofol sedation in a paediatric emergency department: a prospective study. Clin Pediatr 2001; 40: 663–671.
- Aydin Erden, I. Gulsun Pamuk, A. Akinci, et al. Comparison of propofol‐fentanyl with propofol‐fentanyl‐ketamine combination in pediatric patients undergoing interventional radiology procedures. Pediatric Anesthesia 2009; 19: 500-506.
- Kim SH, Kim SI, Ok SY, et al. Opioid sparing effect of low dose ketamine in patients with intravenous patient-controlled analgesia using fentanyl after lumbar spinal fusion surgery. Korean J Anesthesiol. 2013 Jun; 64(6):524-528.
- Michelet D, Hilly J, Skhiri A, et al. Opioid-Sparing Effect of Ketamine in Children: A Meta-Analysis and Trial Sequential Analysis of Published Studies. Paediatr Drugs. 2016 Dec; 18(6):421-433.

The above article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.




