Mental illness and comorbid insomnia: a cross- sectional study of a population of psychiatric in-patients
Lucinda Donaldson and Praveen Kumar Chintapanti
Cite this article as: BJMP 2009:2(2) 36-41
|
|
Abstract Aim : To investigate the self-reported quality of sleep in a population of psychiatric in-patients and to explore any associations between sleep quality and clinical and demographic factors. |
The significance of disturbed subjective sleep quality in the general population is important because of high prevalence rates (of up to 30%)1 and the association with decreased quality of life.2 Poor sleep affects cognitive and physical functioning, and insomnia is associated with a greater risk of falls and accidents,3 higher rates of absenteeism4 and increased health care utilization.4
Insomnia is commonly encountered in primary and secondary care settings, and can be symptomatic of many medical, neurological, substance abuse or primary sleep disorders.
Epidemiological and clinic-based studies consistently demonstrate high rates of psychiatric comorbidity.5,6 Sleep disturbance is an important clinical construct in psychiatry. It represents formal diagnostic criterion in mental illnesses such as affective and anxiety disorders.7,8
Insomnia is broadly defined as the subjective experience of poor or unrefreshing sleep, with some objective evidence of reduced time asleep or delayed sleep-onset. The subjective nature of such complaints remains key, because sleeping is a private event, and there is often no informant history. Furthermore, it is the perceptual aspects of sleep that influence patients’ help-seeking behaviour, such as consultation requests, demands for night sedation, and medication and substance use. It is noteworthy that despite the wide-ranging implications and subjectively distressing nature of this phenomenon, it remains arguably one of the least satisfying symptoms to treat. Seeking a better understanding of the extent and nature of patients’ sleep perception can help optimise appropriate therapeutic strategies.
This is the first study assessing the subjective sleep quality of a sample of psychiatric disordered in-patients in a UK psychiatric hospital setting, using the Pittsburgh Sleep Quality Index (PSQI).9 This study is framed in the context of increasing the awareness of the significance of patients’ complaints of insomnia and addressing the wider psychosocial issues that this raises.
Method
Design: This was a cross-sectional survey of the self-reported quality of sleep in a population of psychiatric in-patients on the acute adult wards of a London psychiatric hospital.
Participants and Procedure:Participants consisted of psychiatric in-patients (ages 18-65) on all five of the acute adult open psychiatric wards of the Highgate Mental Health Centre, London, currently admitted for the assessment or treatment of mental illness. Financial compensation was not provided for any subject.
Subjects were approached on the ward by a member of nursing staff and asked if they were interested in participating in a study about sleep. The researcher was then introduced to explain further details with the aid of the participant information sheet. After a minimum of 24 hours, patients were approached again by the researcher and asked if they were willing to participate. Recruitment of subjects took place if the patient was agreeable to take part and did not meet any of the exclusion criteria (listed below). A scheduled time and date was made with participants in order to obtain written informed consent and to administer the questionnaire. Questionnaire data was collected from each subject by the researcher in a private interview room located on the patient’s psychiatric ward. Demographic and clinical data required from the patient’s medical notes was recorded on the day of sampling. Exclusion criteria were: the presence of severely disturbed behaviour, or having received rapid tranquilisation for such behaviour on the day of sampling; a significant impairment in physical condition (e.g. infection, trauma); a history of a sleep disorder (e.g. obstructive sleep apnoea); the presence of organic illness including dementia; and lack of capacity to give informed consent.
Quality of Sleep:Subjects' quality of sleep was assessed by the administration of the Pittsburgh Sleep Quality Index (PSQI).9 This is one of the most widely used questionnaires employing standardised measures to assess subjective sleep quality in clinical and research settings. It assesses sleep quality and disturbances over a 1-month time interval. 19 individual items are used to generate 7 component scores (with a range of possible subscale scores from 0 to 3): 1) overall subjective sleep quality; 2) sleep latency; 3) sleep duration; 4) habitual sleep efficiency; 5) sleep disturbances 6) use of hypnotic or sedative medication; 7) daytime dysfunction. Higher scores indicate greater sleep disturbances. The sum of the component scores yields a global score (ranging from 0 to 21), which was used as the primary outcome measure in this study. A global PSQI score cut off score of 5 discriminates between good and bad sleepers and the PSQI gives acceptable measures of internal homogeneity, consistency (test-retest reliability) and validity.9,10
Other Variables:Demographic and clinical data recorded concurrently from participant's medical notes included: sex (male/female); age (years); ethnicity (Asian/Black/Mixed//Other); body mass index (BMI) (calculated as the ratio between weight [kilograms] and squared height [metres]); primary psychiatric diagnosis (based on ICD-10 criteria); duration of psychiatric illness (years); past medical history; number of currently prescribed medications; length of admission to date (days); current admission status (informal/detained under Section 2 of the Mental Health Act (MHA) (1983) (this is for a maximum period of 28 days for further assessment)/detained under Section 3 MHA (1983) (this is for a maximum period of 6 months for psychiatric treatment). A further category (detained under another type of section) was dropped as this did not apply to any of the subjects.
Ethics Committee Approval:Ethical and research governance authorisations were granted from Camden and Islington Community Local Research Ethics Committee, and from the North Central London Research Consortium, respectively.
Statistical Analysis:The aim was to compare clinical, demographic and PSQI data between the poor sleepers and good sleepers. The prevalence (%) of poor sleep was determined by the proportion of subjects with global PSQI score of 5 or more. Statistical analyses were predominantly performed using the software package Stata, version 9.2.
Results
Sample Characteristics
77 patients were initially identified as potentially eligible subjects. Of these, 31 (40%) were excluded due to: the presence of disturbed behaviour (n=1); inability to give informed consent (n=9); unwillingness to participate (n=19); absence from ward (either on leave or absent without leave) (n=2).
This left a total of 46 patients who were enrolled in to the study. Subject characteristics are given in Table 1.
Table 1: Demographic and clinical characteristics of study subjects
| Sex, n (%) | |
| Male | 24 (52) |
| Female | 22 (48) |
| Ethnicity, n (%) | |
| Asian | 1 (2) |
| Black | 9 (20) |
| Mixed | 1 (2) |
| Other | 1 (2) |
| White | 34 (74) |
| Current Admission Status, n (%) | |
| Detained under Section 3 MHA | 22 (48) |
| Detained under Section 2 MHA | 5 (11) |
| Informal | 19 (41) |
| Age, years: mean (s.d.) | 38 (11.1) |
| Range | 18-62 |
| Body Mass Index, kg/m²: mean (s.d.) | 25.99 (4.96) |
| Range | 17.9-41.5 |
| Duration of mental illness, years: mean (s.d.) | 10.51 (7.93) |
| Range | 0.17-30 |
| Length of admission, days: mean (s.d.) | 42.43 (63.21) |
| Range | 2-366 |
| Prescribed regular medications, mean (s.d.) | 1.83 (1.05) |
| Range | 0-5 |
| Medical comorbidities, mean (s.d.) | 0.59 (0.98) |
| Range | 0-3 |
s.d.: standard deviation
As defined by ICD-10 criteria, the most common subdivisions of patients’ psychiatric diagnoses in descending order were: paranoid schizophrenia, F20.0, (n=16); emotionally unstable personality disorder, F60.3, (n=6); depressive disorder, F32, (n=6); bipolar affective disorder, F31 (n=5). Other subdivisions of subjects’ diagnoses included: organic mood disorder, F06.3 (n=1); organic personality disorder, F07, (n=1); residual and late onset psychotic disorder due to alcohol use, F10.7, (n=1); persistent delusional disorder, F22, (n=1); acute and transient psychotic disorder, F23, (n=1); unspecified non-organic psychosis, F29, (n=1); post traumatic stress disorder, F43.1, (n=1). One patient was undergoing psychiatric evaluation and therefore had no formal diagnosis.
Medications prescribed regularly were: antipsychotics (for 40% of the total sample of patients), mood stabilizers (16%), antidepressants (14%) and benzodiazepines (7%). In terms of regular night time sedation, two patients out of a total of 46 were prescribed zopiclone and diazepam respectively. Zopiclone was prescribed on an “as required” basis for 15 patients (33% of the total sample).
Overall sleep quality evaluated by the PSQI revealed a mean score of 9.74 (standard deviation= 5.11). Poor sleep quality (defined as a global PSQI score of 5 or more) was present in 36 out of the total of 46 subjects (78% of the sample).
Comparison between good and poor sleepers
Comparison of numerical measurements between the two sleep groups is presented in Table 2. For the normally distributed variables the figures reported for each group are the mean (standard deviation) and the p-value from the t-test. For the non-normally distributed variables the figures reported are the median (inter-quartile range) and the p-value from the Mann-Whitney test.
Table 2: Comparison of demographic and clinical data between good and poor sleepers.
| Variable | Good sleepers |
(total PSQI <5)
Mean (SD)
Poor sleepers
(total PSQI ≥5)
Mean (SD)
P-value
Age (years)41.0 (12.1)37.2 (10.9)0.34BMI (kg/m²)23.0 (2.5)26.7 (5.2)0.15Duration of psychiatric illness (years) (*)10 (8, 12)9.5 (3, 12)0.70Duration of admission (days) (*)44 (13, 111)15 (5, 41)0.06Medications (*)2 (1, 3)2 (1, 4)0.55Psychiatric medications (*)1.5 (1, 2)2 (1, 2)0.68
(*) Median (Inter-quartile range) reported. Analysis performed using Mann-Whitney test
The results indicate that there was no strong evidence of a statistically significant difference between good and poor sleepers for any of the variables examined. However, there was a possible difference for duration of admission, although this result was only of borderline statistical significance (p=0.06). The results indicate a median duration of admission of 44 days for good sleepers and 15 days for poor sleepers.
The difference between sleep groups for the categorical variables was examined using Fisher’s exact test. Results, presented in Table 3, show the number (and percentage) of subjects falling into each category, with the p-value indicating the significance of the results.
Table 3: Comparison of categorical data between good and poor sleepers
| Variable | Group | Good sleepers (total PSQI <5) N (%) | Poor sleepers (total PSQI ≥5) N (%) | P-value |
Sex | Female | 3 (30%) | 19 (53%) | 0.29 |
| Male | 7 (70%) | 17 (47%) | ||
Admission status | Section 3 | 9 (90%) | 18 (50%) | 0.01 |
| Section 2 | 0 (0%) | 5 (14%) | ||
| Informal | 1 (10%) | 13 (36%) | ||
Physical comorbidities | None | 7 (70%) | 24 (67%) | 1.00 |
| 1+ | 3 (30%) | 12 (33%) |
There was a significant difference between sleep groups with regard to their admission status. Almost all (90%) of the good sleepers were detained under Section 3 MHA (1983), whilst this applied to only half of those in the poor sleepers group. Being detained under Section 2 MHA and informal admission were more commonly found amongst those categorised as poor sleepers.
There was no significant difference between groups in terms of sex or the presence of physical comorbidities.
The final set of analyses compared the differences between groups for the PSQI measures, and the results are summarised in Table 4. The figures reported are the mean (standard deviation) score for each group. For the individual components the Mann-Whitney test was used to compare between groups, and the p-values from this analysis are reported. For the PSQI total score, the unequal variance t-test was used to compare between groups.
Table 4: Comparison of PSQI measures between the good and poor sleepers
| Good sleepers (total PSQI <5) Mean (SD) | Poor sleepers (total PSQI ≥5) Mean (SD) | P-value | |
| PSQI C1 score (quality) (*) | 0.2 (0.84) | 1.6 (0.9) | <0.001 |
| PSQI C2 score (latency) (*) | 0.9 (1.0) | 1.8 (1.0) | 0.02 |
| PSQI C3 score (duration) (*) | 0.1 (0.3) | 1.7 (1.3) | 0.002 |
| PSQI C4 score (efficiency) (*) | 0.1 (0.3) | 1.7 (1.3) | 0.001 |
| PSQI C5 score (disturbances) (*) | 0.7 (0.5) | 1.4 (0.6) | 0.003 |
| PSQI C6 score (sedatives) (*) | 0.4 (1.0) | 1.5 (1.4) | 0.04 |
| PSQI C7 score (daytime dysfunction) (*) | 0.7 (0.9) | 2.1 (0.8) | 0.004 |
| PSQI total | 3.1 (1.3) | 11.6 (4.1) | <0.001 |
(*) Analysis performed using Mann-Whitney test
There was a statistically significant difference between good and poor sleepers for all PSQI components and for the PSQI total. The PSQI component values and PSQI total scores for poor sleepers were significantly higher than for good sleepers.
A profile of the mean PSQI individual component scores between the two groups (good sleepers versus poor sleepers) is displayed in Figure 1.
Figure 1: Mean component PSQI scores of good and bad sleepers
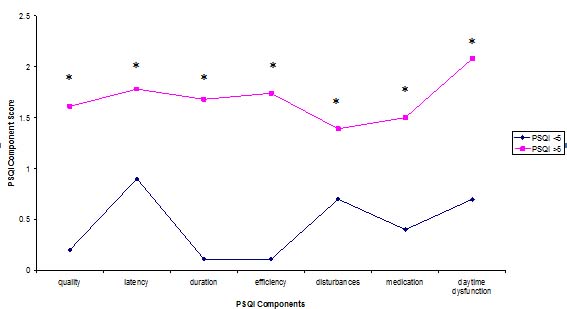
Profiles of the PSQI represent group differences of individual component scores. Mann-Whitney test, *p<0.05).
Subjective Patient Comments
The PSQI also comprises an open ended question, providing subjects with the opportunity to cite “other” (subjective) reasons for difficult sleep. The most common response was anxiety (n=8). Other examples included: medication alterations (n=3); environmental noise (n=2); “thinking excessively” (n=1); “a desire to be creative” (n=1); hard mattress (n=1); “food eaten” (n=1); “sedentary lifestyle” (n=1); alcohol (n=1); hunger (n=1); asthma (n=1); symptoms of the menopause (n=1); and “voices”(n=1).
Discussion
Main Results
This is the first study to examine the subjective quality of sleep among a population of psychiatric in-patients in the UK. The prevalence of poor sleep, as defined by a cut off PSQI score of 5 or more, was present in 78% of the patients sampled. Patients detained under Section 3 MHA (1983) were more likely to report sleeping well when compared to informal patients or those detained under section 2 MHA (1983). There was some evidence of good subjective sleep quality being related to a longer duration of admission, but this requires further investigation.
There were no significant differences between good and poor sleepers for any of the other demographic and clinical variables studied, including age, body mass index, duration of psychiatric illness, number of prescribed medications, sex, and physical comorbidities.
Individual PSQI component scores and global scores were significantly lower for good sleepers compared to poor sleepers. This would be expected given that higher scores indicate more severe sleep complaints, and this supports the consistency of the PSQI as a research instrument.
Factors Affecting Sleep
In-patients’ disturbed sleep may be caused by a variety of exogenous factors such as unfamiliar surroundings, environmental noise, bright lighting and staff interactions or monitoring. Physical and psychological factors, such as the side-effects of medication and substance use, may also have a detrimental effect on sleep quality. In the added presence of a psychiatric disorder, each of these factors may act synergistically on the relationship between mental illness and sleep. Despite substantial research supporting the robust associations between insomnia and comorbid conditions, specific mechanisms linking sleep, medical and psychiatric factors have not been well established.
Sleep complaints may represent early symptoms and risk factors for new episodes of mental illness rather than simply representing phenomena secondary to experience of mental illness. For example, longitudinal studies have found insomnia to be a substantial risk factor for the development of a depressive disorder5,11,12 and the risk for developing new anxiety disorders and alcohol abuse is also greater for insomniacs.6
Stepanski & Rybarczyk13 present research arguing against the more traditional conceptualisation of insomnia as simply a consequence of another disorder. They propose the need for a revised model to understand insomnia that is comorbid with medical and/or psychiatric illness. Abnormalities of the hypothalamic-pituitary-adrenal (HPA) axis may represent the underlying pathophysiological process in many chronic insomnia patients.14 This may signify a common risk factor for insomnia and depression, thus predisposing the individual to a vulnerability to both conditions.15
In this study, detained patients (under Section 3 MHA (1983)) were significantly more likely to be classified as good sleepers. A suggestion for this finding could be that these patients may be less resisting of remaining and sleeping on the ward due to the involuntary nature of their admission. Alternatively these patients may represent the group with the most severe mental illnesses and with the least insight, and therefore less able to accurately recall their (poor) sleeping habits over the previous month.
There was also a potential association between longer admission status and better sleep quality. Explanations for this observation might include: patients’ acceptance over time of their admission and the consequent conditioning to, and familiarisation with, the ward environment; achievement of stability in mental state over time; or the adaptation of the perception of sleep quality to the sleep disturbances that accompany mental illness.
Limitations
This study is based on cross-sectional data and the relationship between the course of mental illness and sleep perception cannot be determined. In order to verify the direction of causality, it is necessary to demonstrate longitudinally that improvement in symptom severity is accompanied by an increase in subjective sleep quality.
This study was not designed to look at the prevalence of poor sleep across the different classes of psychiatric illnesses and dual diagnoses were not considered. It did not measure psychopathology or self-reported psychological distress. Possible confounding factors were not taken in to account, such as concurrent use of caffeine, alcohol, nicotine, illicit substances, hypnotics or other medications known to affect sleep.
The PSQI measures sleep quality averaged over the previous month. In cases where patients had only very recently been admitted to hospital, measurements would have been unlikely to accurately reflect the perspective of an in-patient’s experience. The mean length of admission for this population however was longer than one month (42 days).
These results were drawn from a small sample, with a fairly high proportion of excluded patients (40%). This may explain why this study did not identify factors previously found to more frequently affect sleep adversely such as female gender, the elderly and those with chronic medical conditions.16 In addition the population sample has little ethnic diversity which limits the generalisability of the results.
Implications
This study found that the prevalence of poor sleep quality was more common than previously reported in the general population17 and more comparable to the higher rates reported in similar patient populations. Two previous studies investigating subjective sleep quality using the PSQI, found prevalence rates of poor sleepers to be 45.5%18, and 91.22%19 among a population of schizophrenia patients and psychiatric in-patients respectively.
Complaints of poor sleep are important for diagnostic purposes and also raise the need to address the adequacy of therapeutic strategies, given the consequent adverse impact on patients’ mental state, physical health, daytime function and quality of life.
Improving Sleep
Hypnotics such as benzodiazepines and benzodiazepine receptor agonists can be efficacious for the treatment of insomnia.20,21 However the clinical benefits must be weighed against well known adverse effects, such as daytime sedation, agitation, memory impairment, confusion and ataxia. This, together with the recommendation that hypnotics should only be used for short periods of time because of the risk of drug tolerance and dependence,22 highlights the need for suitable non-pharmacological alternatives.
Recent reviews support the notion of the effectiveness of Cognitive Behavioural Therapy for insomnia in the treatment of people with psychiatric or medical conditions.13,23 Modified, lower cost education initiatives to promote good sleep could be employed by utilising the skills of the mental health professionals caring for patients on the ward, supplemented by the provision of clear written material.
Environmental variables to consider include adherence to regular ward routines including bedtime and awakening times, attention to ward layout and design (including the provision individual bedrooms), lighting, ambient noise, temperature, and the provision of comfortable mattresses and appropriate bed linen. Medication scheduling times, regular medication reviews, and avoidance of non-prescribed substances such as caffeine, alcohol and illicit substances are also important. Physical health problems, pain and psychological distress should be optimally managed. Moderate intensity exercise programs have also been found to bring about significant improvements in self-rated sleep quality.24 Finally, increased staff awareness and sensitivity to the sleep problems on the ward, supplemented with objective recording of such disturbances, would be informative in gaining a further understanding of patients’ insomnia experiences.
Future Directions for Research
The PSQI is simple and inexpensive to perform. Results could be followed longitudinally in order to examine the course of sleep problems throughout an episode of acute mental illness, or to examine the effects of specific therapeutic interventions for sleep disorders. Sleep diaries have been shown to provide reliable estimates of subjective sleep parameters25 and could be used as an adjunct to the PSQI. Ideally, concomitant objective measures such as polysomnography or wrist actigraphy (which detects physical motion), as well as cognitive and behavioural measures could be used to provide additional data.
This study represents the first attempt to examine the degree of self-reported poor sleep quality in a UK-based population of psychiatric in-patients and results suggest unsatisfactory sleep is a common finding. Large prospective longitudinal studies of sleep quality with control for confounding factors are needed to confirm the high prevalence rates in psychiatric in-patients. Studies comparing psychiatric patients with healthy controls, and also with insomniacs without psychiatric comorbidity, would further clarify the role of psychopathology in sleep disturbance.
| ACKNOWLEDGEMENTS With thanks to Mr Paul Bassett, Statistical Consultant COMPETING INTERESTS None Declared AUTHOR DETAILS LUCINDA DONALDSON, BSc, MB BS, MRCPsych, Specialty Registrar, Barnet, Enfield and Haringey Mental Health Trust, United Kingdom PRAVEEN KUMAR CHINTAPANTI, MB BS, DPM, MRCPsych, Consultant Psychiatrist, Camden and Islington NHS Foundation Trust, United Kingdom CORRESPONDENCE: LUCINDA DONALDSON, Specialty Registrar, South West Complex Mental Health Team, 7th Floor Premier House, 112 Station Road, Edgware, Middlesex HA8 7BJ, United Kingdom Email: lucindadonaldson@yahoo.co.uk |
References
- 1. Ancoli-Israel S, Roth T. Characteristics of insomnia in the United States: results of the 1991 National Sleep Foundation Survey. I. Sleep 1999;22 Suppl 2:S347-53.
- 2. Leger D, Scheuermaier K, Philip P, et al. SF-36: Evaluation of quality of life in severe and mild insomniacs compared with good sleepers. Psychosom Med 2001;63:49-55.
- 3. Roth T. Prevalence, associated risks, and treatment patterns of insomnia. J Clin Psychiatry 2005;66 suppl 9:10-13.
- 4. Leger D, Guilleminault C, Bader G, et al. Medical and socio-professional impact of insomnia. Sleep 2002;25(6):625-629.
- 5. Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA 1989;262:1479-84.
- 6. McCall WV. A psychiatric perspective on insomnia. J Clin Psychiatry 2001;62 Suppl 10:27-32.
- 7. World Health Organisation. The ICD-10 Classification of Mental and Behavioural Disorders: Diagnostic Criteria for Research. Geneva: World Health Organisation, 1993.
- 8. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (4th edn) (DSM-IV). Washington, DC: American Psychiatric Association, 1994.
- 9. Buysse DJ, Reynolds CF 3rd, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989;28:193-213.
- 10. Doi Y, Minowa M, Uchiyama M, et al. Psychometric assessment of subjective sleep quality using the Japanese version of the Pittsburgh Sleep Quality Index (PSQI-J) in psychiatric disordered and control subjects. Psychiatry Res 2000;97:165-172.
- 11. Breslau N, Roth T, Rosenthal L, et al. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biol Psychiatry 1996;39:411-418.
- 12. Chang PP, Ford DE, Mead LA, et al. Insomnia in young men and subsequent depression. The Johns Hopkins Precursors Study. Am J Epidemiol 1997;146:105-114.
- 13. Stepanski EJ, Rybarczyk B. Emerging research on the treatment and etiology of secondary of cormorbid insomnia. Sleep Med Rev 2006;10:7-18.
- 14. Richardson GS, Roth T. Future directions in the management of insomnia. J Clin Psychiatry 2001;62 suppl 10:39-45.
- 15. Roth T, Roehrs T. Insomnia: Epidemiology, Characteristics, and Consequences. Clin Cornerstone 2003;5(3):5-15.
- 16. Morin CM, Hauri PJ, Espie CA, et al. Nonpharmacologic treatment of chronic insomnia: an American Academy of Sleep Medicine review. Sleep 1999;22:1134-56.
- 17. Doi Y, Minowa M, Uchiyama M, et al. Subjective sleep quality and sleep problems in the general Japanese adult population. Psychiatry Clin Neurosci 2001; 55(3): 213-215.
- 18. Ritsner M, Kurs R, Ponizovosky A, et al. Perceived quality of life in schizophrenia: Relationships to sleep quality. Qual Life Res 2004;13:783-791.
- 19. Prieto-Rincón D, Echeto-Inciarte S, Faneite-Hernández P, et al. [Quality of sleep in hospitalized psychiatric patients]. Invest Clin 2006;47:5-16.
- 20. Nowell PM, Mazumdar S, Buysse DJ, et al. Benzopdidazepines and zolpidem for chronic insomnia: a meta-analysis of treatment efficacy. JAMA 1997;278:2170-7.
- 21. Holbrook AM, Crowther R, Lotter A, et al. Meta-analysis of benzopdiazepine use in the treatment of insomnia. CMAJ, 2000; 162: 225-233.
- 22. National Institute for Health and Clinical Excellence. Guidance on the use of zaleplon, zolpidem and zopiclone for the short-term management of insomnia. London: NICE, 2004. (Technology Appraisal 77.) Available from: URL: http://www.nice.org.uk/TA077guidance
- 23. Smith MT, Huang MI, Manber R. Cognitive behavior therapy for chronic insomnia occurring within the context of medical and psychiatric disorders. Clin Psychol Rev 2005;25:559-92.
- 24. King AC, Oman RF, Brassington GS, Bliwise DL, Haskell WL. Moderate-Intensity Exercise and Self-rated Quality of Sleep in Older Adults. A Randomized Controlled Trial. JAMA 1997;277:32-37.
- 25. Coates TJ, Killen JD, George J, et al. Estimating sleep parameters: A multitrait-multimethod analysis. J Consult Clin Psychol 1982;50:345-52.

The above article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.




