Colonic Metastasis from a Breast Carcinoma, an Unusual Colonoscopic Finding
Wadah Ali, Zakir K Mohamed and D Thekkinkattil
Cite this article as: BJMP 2016;9(1):a901
|
|
Abstract Breast cancer is a leading cause of cancer deaths in females in the UK. Distant metastases are the commonest cause of death and the lung, liver and bones are the most common sites. Metastases to the gastrointestinal (GI) tract are rare with colonic metastases even rarer and as such may pose a diagnostic challenge. They are much less common than primary intestinal tumours. Here, we report an interesting case of a patient who presented with colonic metastasis over six years following treatment of a breast carcinoma. Keywords: Breast cancer, colon metastasis, colonoscopy |
CASE REPORT
A 61-year-old lady underwent a modified radical mastectomy and axillary clearance in 2008 for a carcinoma of the left breast. Histopathology examination revealed two tumours within the left breast; a 16mm Grade 2 lobular carcinoma with probable vascular invasion and a 9mm Grade 1 infiltrating ductal carcinoma with no vascular invasion. She had clear resection margins. 21 out of 34 removed lymph nodes were positive for metastatic deposits. The tumour was oestrogen receptor positive and HER2 negative. She was staged as T1 N3a Mx and the tumour had a Nottingham Prognostic Index of 5.32. Metastatic workup revealed no distant metastasis.
Postoperatively, she required aspiration of a seroma but her recovery was otherwise satisfactory. She received adjuvant chemotherapy in the form of three cycles of Fluorouracil, Epirubicin and Cyclophosphamide and 3 cycles of Docetaxel. In addition, she had postoperative radiotherapy to the chest wall and supraclavicular fossa (40 Gy in 15 Fractions over 3 weeks) and hormonal therapy with Letrozole 2.5mg once daily.
The patient opted to undergo a prophylactic right mastectomy in 2010. She was regular in follow up and appeared to be free of disease recurrence for 6 years.
Her past surgical history included abdominal hysterectomy and bilateral salpingo-ophorectomy for fibroid disease as well as varicose vein stripping. She is a non-smoker and doesn’t consume alcohol. She had a family history of colon and cervical cancer in her uncle and sister respectively.
The patient visited the surgical outpatient clinic complaining of abdominal cramps, altered bowel habits and fatigue of a few months duration. There was no associated rectal bleeding, haematemesis, melaena, weight loss or urinary symptoms. Physical examination was unremarkable but she was noted to have gradually worsening renal function. Her symptoms were at first attributed to side effects of intravenous antibiotic treatment, which she received during an admission for cellulitis. She had already undergone an upper GI endoscopy which showed oesophagitis and ulceration; biopsies were within normal limits. She received treatment with proton pump inhibitors but her symptoms persisted.
A non-contrast abdominal CT scan was done, on account of her poor renal function, which showed bilateral hydronephrosis and thickening of the postero-superior aspect of the bladder wall. Considering the limitations of the non-contrast study, there were no other abnormalities. A colonoscopy was also done to investigate her altered bowel habit and it revealed a benign-looking stricture in the sigmoid about 25cm from the anal verge which was easily bypassed by the scope.
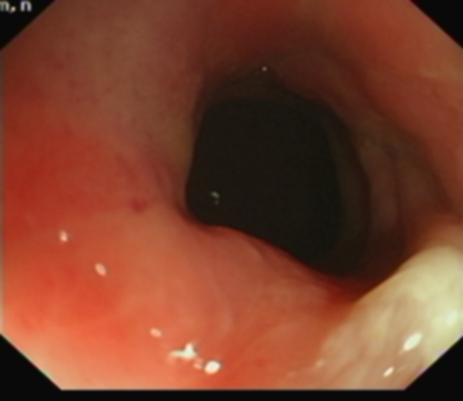
Figure 1. Benign stricture on flexible sigmoidoscopy
Biopsies of the sigmoid stricture showed an infiltrate of small to medium sized tumour cells in the submucosa, which had an Indian file pattern. They were positive for AE1/AE3 (pancytokeratins) and negative for CD68. They were positive for CK7 and negative for CK20, strongly positive for oestrogen receptors and HER2 negative. Taken in conjunction with the patient’s past history of an invasive lobular carcinoma of the breast, the appearance was consistent with a metastatic lobular carcinoma.
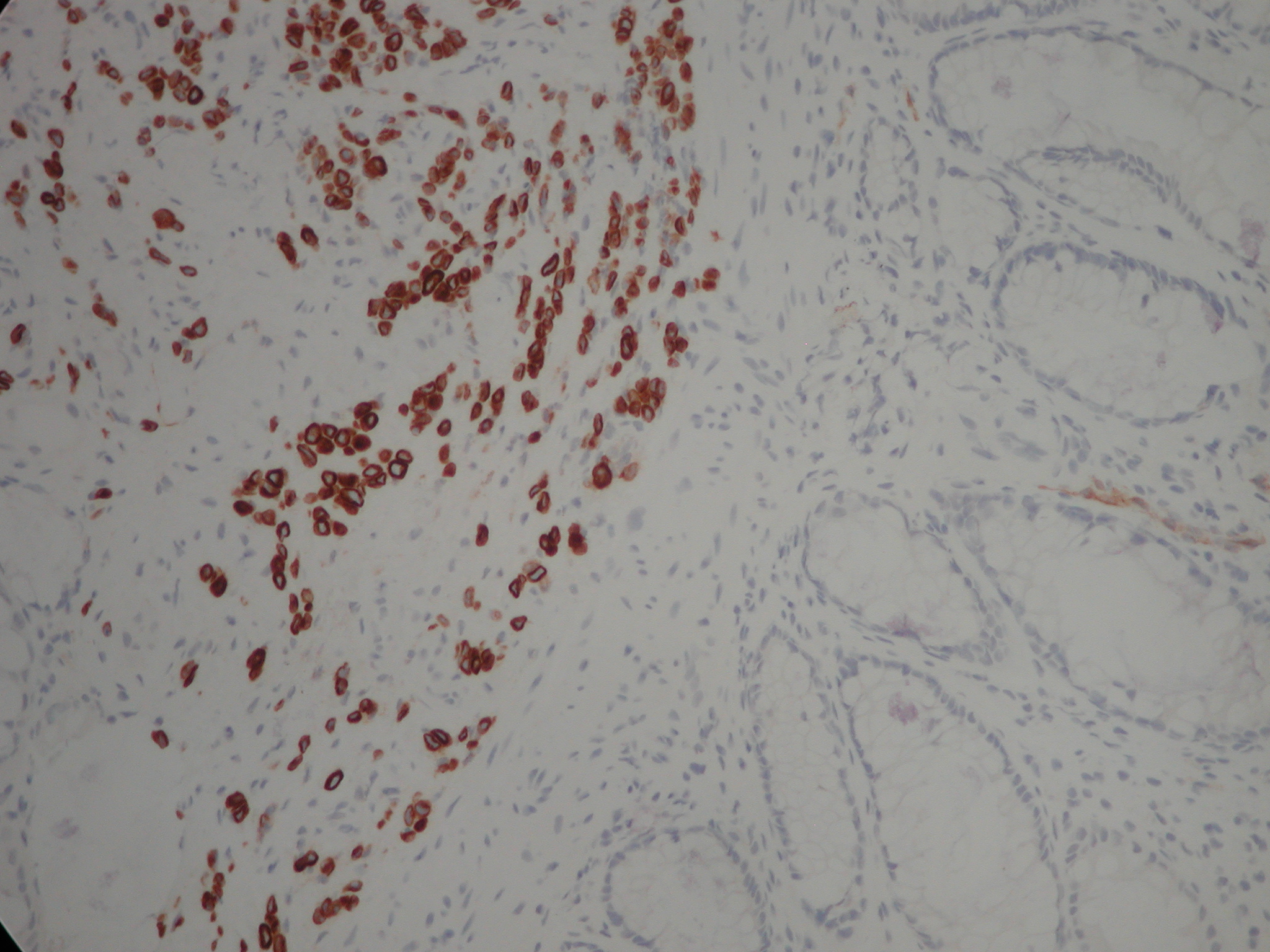
Figure 2. Clusters and cords of cells with positive cytoplasm for the cytokeratin immunostain CK7. Although the classical ‘Indian filing’ of lobular carcinoma is not well seen, the image clearly demonstrates that the large bowel glands are negative (normally CK20+, CK7-) and that the infiltrate is beneath the glandular mucosa (i.e. not originating from dysplastic glands within the mucosa and raising the possibility of infiltration from outside the bowel wall). The magnification is x200. Lobular carcinoma is usually CK7 +, CK20 -, ER +.
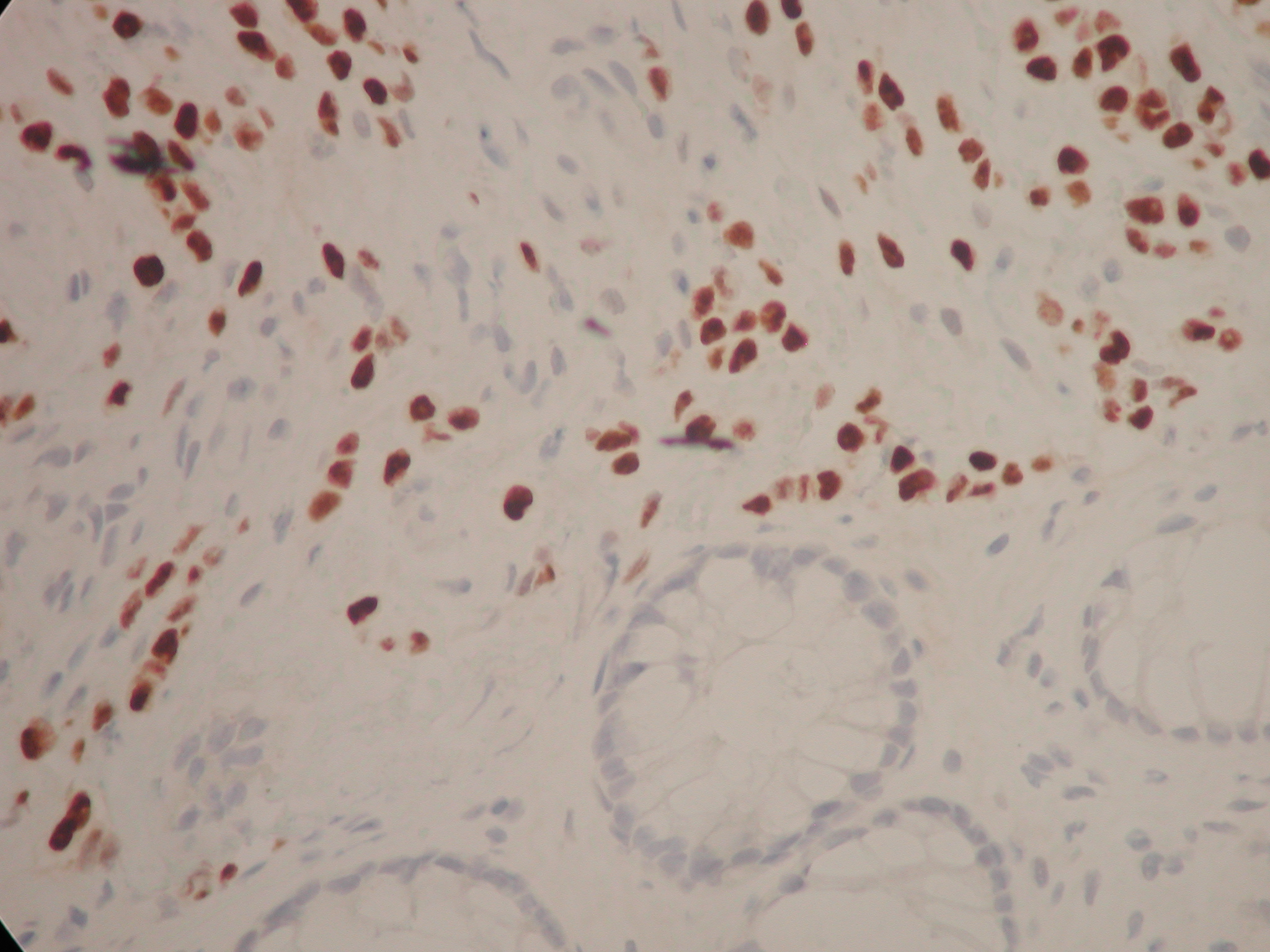
Figure 3. The same cells with their nuclei staining positively with an immunostain to oestrogen receptors. There are a few short chains of ‘Indian filing’ with the cells appearing rather rectangular in shape with straight margins. You can make out slight ‘moulding’ of the nuclei as they press against one another. The magnification is x 400.
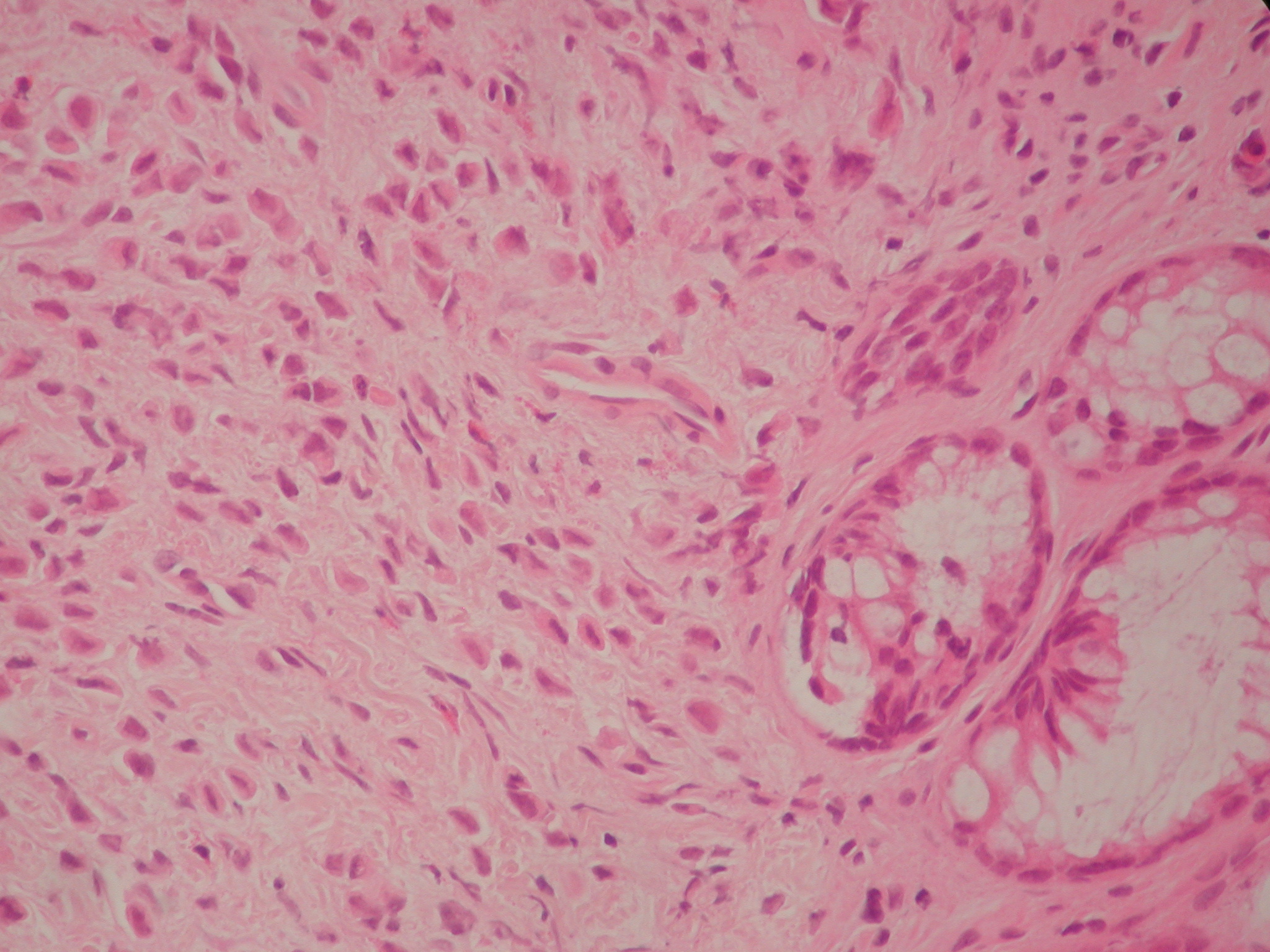
Figure 4. Haematoxylin and Eosin section at 400 magnification. This shows a diffuse infiltrate of single cells with eccentric nuclei.
The patient required a right nephrostomy and a cystoscopy with left double J ureteric stent insertion to address her hydronephrosis and deteriorating renal function before undergoing restaging of her disease.
DISCUSSION
In patients with history of breast cancer, isolated GI metastases are less common than benign disease processes or second primaries of the GI tract.1.2 In a retrospective review, 73 out of 12001 cases of breast cancer had gastrointestinal metastases, out of which 24 were to the colorectum3 and invasive lobular carcinoma was the commonest histological subtype. 3.4 However, sixteen percent of patients with breast cancer have GI metastases at postmortem examination1.
There might be a long interval of time between the diagnosis of breast cancer and development of gastrointestinal metastasis which together with their rare occurrence and nonspecific clinical and radiological manifestations adds to the diagnostic challenge. The median interval between the diagnosis and the development of GI metastasis was reported to be 6 years (range 0.25 to 12.5 years) by Schwarz et al 5with 25 years being the longest reported in the literature.6 Because of this long interval the history of a primary breast cancer can be missed. This also highlights the importance of long term follow up and maintaining an index of suspicion when these patients develop GI symptoms.
In our case, the interval between the diagnosis of breast cancer and colonic metastasis was 81 months. Her GI symptoms were initially attributed to side effects of antibiotic treatment for cellulitis and dyspepsia before investigating her with a colonoscopy. Even at colonoscopy the appearance was that of a smooth benign-looking stricture which did not seem to harbour any sinister pathology
Histological examination is probably the most reliable tool to make a diagnosis and it is prudent in such cases to compare the specimen with the original breast tumour. In this case, there were two primary tumours; an invasive ductal carcinoma as well as a lobular carcinoma but the metastatic disease favoured the lobular component, which is consistent with other published reports in the literature. The reasons why metastases favour lobular carcinoma are poorly understood. One explanation is the loss of E-cadherin expression, a molecule involved in cellular adhesion, in invasive lobular carcinoma7. A similar case in which the primary was a mixed ductal and lobular type with lobular subtype colonic metastasis was reported by Uygun et al.8 Immunohistochemistry can also help in establishing a diagnosis. Metastatic breast cancers tend to be positive for Oestrogen or Progesterone receptors as well as Gross Cystic Disease Fluid Protein-15.9, 10 It is, however, worth noting that primary colonic cancers can be oestrogen receptor positive in 30 to 70% of cases.11
Accurate histopathological diagnosis probably saved our patient an unnecessary surgical treatment for a primary colonic neoplasm as the main focus of her treatment should be systemic therapy for metastatic breast cancer.
CONCLUSION
GI tract metastases from breast cancer are a rare occurrence. The patients may present after a long interval from the original diagnosis and the clinical and radiological features are nonspecific with the diagnosis often established on histological examination. Moreover, the history of breast cancer may not be elicited in all cases and these patients may present to a gastroenterologist or colorectal surgeon rather than a breast surgeon or oncologist. Therefore, remaining vigilant to this possibility is advised in any patient with a history of breast cancer who presents with unexplained GI symptoms.
|
Competing Interests None declared Author Details WADAH ALI MBBS MRCS(Glasg) CABHS, General Surgery Registrar, Pilgrim Hospital, Boston, Lincolnshire. ZAKIR K MOHAMED MRCSEd MSc FRCSEd FRCSEng, Consultant Colorectal Surgeon, Pilgrim Hospital, Boston, Lincolnshire. DINESH THEKKINKATTIL MS MD FRCS, Consultant Breast and Oncoplastic Surgeon, Pilgrim Hospital, Boston, Lincolnshire. CORRESPONDENCE: WADAH ALI MBBS MRCS(Glasg) CABHS: General Surgery Registrar, Pilgrim Hospital, Boston, Lincolnshire, United Kingdom. Email: Wadah.Ali@ULH.nhs.uk |
References
- Cifuentes N, Pickren JW: Metastases from carcinoma of mammary gland: an autopsy study. J Surg Oncol 1979, 11:193–205.
- Yokota T, Kunii Y, Kagami M, Yamada Y, Takahashi M, Kikuchi S, et al: Metastatic breast carcinoma masquerading as primary colon cancer. Am J Gastroenterol 2000, 95:3014–3016.
- McLemore EC, Pockaj BA, Reynolds C, Gray RJ, Hernandez JL, Grant CS, Donohue JH:Breas cancer: presentation and intervention in women with gastrointestinal metastasis and carcinomatosis. Ann Surg Oncol 2005, 12:886-894.
- Taal BG, den Hartog Jager FC, Steinmetz R, Peterse H: The spectrum of gastrointestinal metastases of breast carcinoma: II. The colon and rectum. Gastrointest Endosc 1992, 38:136-141
- Schwarz RE, Klimstra DS, Turnbull AD: Metastatic breast cancer masquerading as gastrointestinal primary. Am J Gastroenterol 1998, 93:111–114.
- Winston CB, Hadar O, Teitcher JB, Caravelli JF, Sklarin NT, Panicek DM, Liberman L: Metastatic lobular carcinoma of the breast: patterns of spread in the chest, abdomen, and pelvis on CT. AJR Am J Roentgenol 2000, 175:795-800.
- Sastre-Grau X, Jouve M, Asselain B et al: Infiltrating lobular carcinoma of the breast. Clinicopathologic analysis of 975 cases with reference to data on conservative therapy and metastatic patterns. Cancer 1996, 77: 113–120.
- Uygun K, Kocak Z, Altaner S, Cicin I, Tokatli F, Uzal C: Colonic Metastasis from Carcinoma of the Breast that Mimics a Primary Intestinal Cancer. Yonsei Medical Journal 2006, 47(4):578-582.
- Monteagudo C, Merino MJ, LaPorte N, Neumann RD: Value of gross cystic disease fluid protein-15 in distinguishing metastatic breast carcinomas among poorly diffentiated neoplasms involving the ovary. Hum Pathol 1991, 22:368–372.
- Bracali G, Caracino AM, Rossodivita F, Bianchi C, Loli MG, Bracali M: Estrogen and progesterone receptors in human colorectal tumour cells (study of 70 cases). Int J Biol Markers 1988, 3:41–48.
- Sastre-Grau X, Jouve M, Asselain B et al: Infiltrating lobular carcinoma of the breast. Clinicopathologic analysis of 975 cases with reference to data on conservative therapy and metastatic patterns. Cancer 1996, 77: 113–120.

The above article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.




